1. Introduction
Natural products have long occupied a central, almost irreplaceable, position in drug discovery. Even in the age of combinatorial chemistry and rational drug design, a significant proportion of approved therapeutics either originate directly from natural compounds or are structurally inspired by them (Cragg & Newman, 2013; Newman & Cragg, 2016). This continued reliance is not accidental. The stereochemical richness, scaffold diversity, and biosynthetic refinement inherent to secondary metabolites often exceed what is typically achieved through purely synthetic libraries (Kumar & Pandey, 2013). Such structural complexity enables natural products to engage biological targets with unusual specificity, sometimes modulating redox-sensitive enzymes, mitochondrial complexes, and regulatory proteins in ways that small, planar synthetic molecules cannot easily replicate.
The urgency of rediscovering and systematically evaluating natural products is amplified by the escalating crisis of antimicrobial resistance. Gram-negative bacteria, in particular, pose a formidable threat due to their impermeable outer membranes, efflux pumps, and diverse enzymatic resistance strategies (Breijyeh et al., 2020; Silver, 2011). Conventional antibacterial pipelines have struggled to keep pace with these adaptive mechanisms. As a result, there has been renewed attention toward plant- and microbe-derived metabolites that evolved within competitive ecological niches and often target nontraditional pathways, including virulence regulation and metabolic adaptation (Núñez-Montero & Barrientos, 2018; Ríos & Recio, 2005). Botanical bioactives and related secondary metabolites frequently display multitarget effects, which may reduce the probability of rapid resistance development compared with single-target synthetic agents (Isman, 2006).
Oxidative stress represents another major therapeutic frontier where natural products demonstrate profound relevance. Reactive oxygen species (ROS) are unavoidable by-products of cellular metabolism, yet excessive accumulation disrupts lipids, proteins, and DNA, contributing to carcinogenesis, inflammation, and degenerative disorders. Antioxidant compounds derived from plants and microorganisms can buffer these redox imbalances either through direct radical scavenging or by enhancing endogenous defense systems (Pisoschi & Pop, 2015). Flavonoids, for example, exemplify a class of structurally diverse natural antioxidants whose bioactivities extend beyond radical neutralization to include modulation of signaling pathways and enzyme inhibition (Kumar & Pandey, 2013).
Interestingly, the biological role of antioxidants is not always straightforward. At certain concentrations or under particular cellular conditions, compounds traditionally classified as antioxidants may exert prooxidant effects, selectively increasing oxidative stress in pathological cells. This duality is especially relevant in oncology, where mitochondrial dysfunction and metabolic reprogramming are hallmarks of tumor progression (Ralph et al., 2010). Targeting mitochondrial bioenergetics has therefore emerged as a promising strategy in anticancer therapy.
Within this context, inhibitors of mitochondrial complex I (NADH–ubiquinone reductase) are of particular interest. Complex I plays a pivotal role in oxidative phosphorylation, and its inhibition can precipitate ATP depletion and apoptosis (Degli Esposti, 1998). Certain plant-derived metabolites, such as annonaceous acetogenins, have demonstrated potent complex I inhibitory activity, contributing to their cytotoxic properties (McLaughlin, 2008). However, such potency is accompanied by safety concerns, as chronic mitochondrial inhibition may also produce neurotoxic consequences. This delicate balance between efficacy and toxicity underscores the need for systematic evidence synthesis rather than isolated experimental interpretation.
Natural products also intersect with microbial bioenergetics and virulence regulation. For instance, inhibitors targeting components of the cytochrome bc1 complex have demonstrated antimicrobial and antifungal potential (Fisher & Meunier, 2008). Strobilurin fungicides, derived from natural fungal metabolites, illustrate how ecological chemical warfare can inspire modern agrochemical and pharmaceutical development (Bartlett et al., 2002). These examples highlight how bioactive natural products can disrupt pathogen energy metabolism while sparing host systems, although resistance mechanisms may eventually emerge.
Beyond antibacterial and anticancer applications, the discovery pipeline for natural products relies heavily on robust screening methodologies. Early-stage cytotoxicity and bioactivity evaluations often utilize general assays such as the brine shrimp lethality test, which provides a rapid and cost-effective method for identifying promising extracts (Meyer et al., 1982). Following preliminary screening, sophisticated isolation and characterization techniques enable purification and structural elucidation of active constituents (Sarker & Nahar, 2012). Advances in chromatography, spectroscopy, and metabolomics have dramatically improved access to complex natural matrices.
Microbial endophytes and environmental microorganisms represent particularly rich sources of chemically novel metabolites. Bioprospecting efforts have revealed that fungi and bacteria residing within plants frequently produce unique secondary metabolites with antimicrobial and cytotoxic potential (Strobel & Daisy, 2003). Such discoveries reinforce the ecological dimension of drug discovery: bioactive compounds are often evolutionary responses to environmental stressors or interspecies competition.
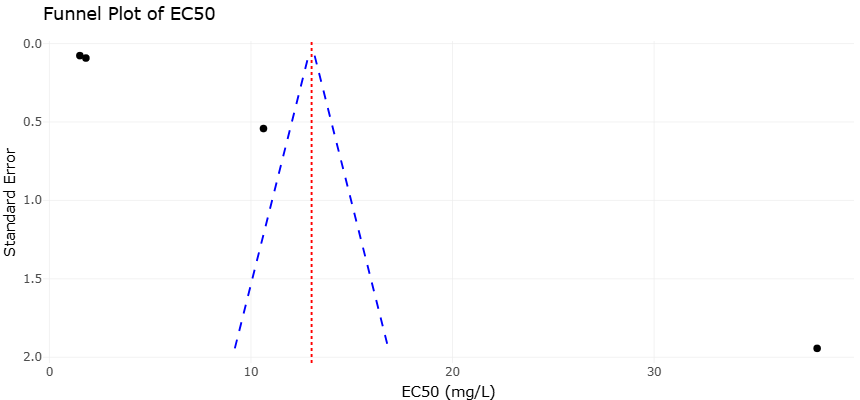
Given the expanding body of experimental data on antioxidants, mitochondrial inhibitors, and antivirulence agents, rigorous quantitative synthesis has become increasingly important. Systematic reviews and meta-analyses provide structured frameworks for evaluating heterogeneous findings and identifying consistent therapeutic signals (Borenstein et al., 2009). Statistical approaches for assessing between-study variability, such as the I² metric, help determine whether observed differences reflect methodological diversity or true biological heterogeneity (Higgins et al., 2003). Moreover, graphical and statistical tools can detect potential publication bias, ensuring that conclusions are not disproportionately influenced by selective reporting (Egger et al., 1997).
In light of the persistent challenges posed by antimicrobial resistance, oxidative stress–related disorders, and mitochondrial dysfunction, natural products remain indispensable reservoirs of pharmacological innovation. Their capacity to function as antioxidants, enzyme inhibitors, and modulators of microbial virulence underscores their multidimensional therapeutic potential. However, translating these bioactivities into clinically viable interventions requires careful integration of mechanistic insight, safety evaluation, and quantitative evidence synthesis.
This systematic review and meta-analysis, therefore, aim to synthesize current evidence on bioactive natural products targeting oxidative stress, mitochondrial function, and microbial virulence. By integrating biochemical, pharmacological, and statistical perspectives, the present study seeks to clarify not only the magnitude of observed effects but also the reliability and translational feasibility of these compounds within contemporary biomedical frameworks.