1. Introduction
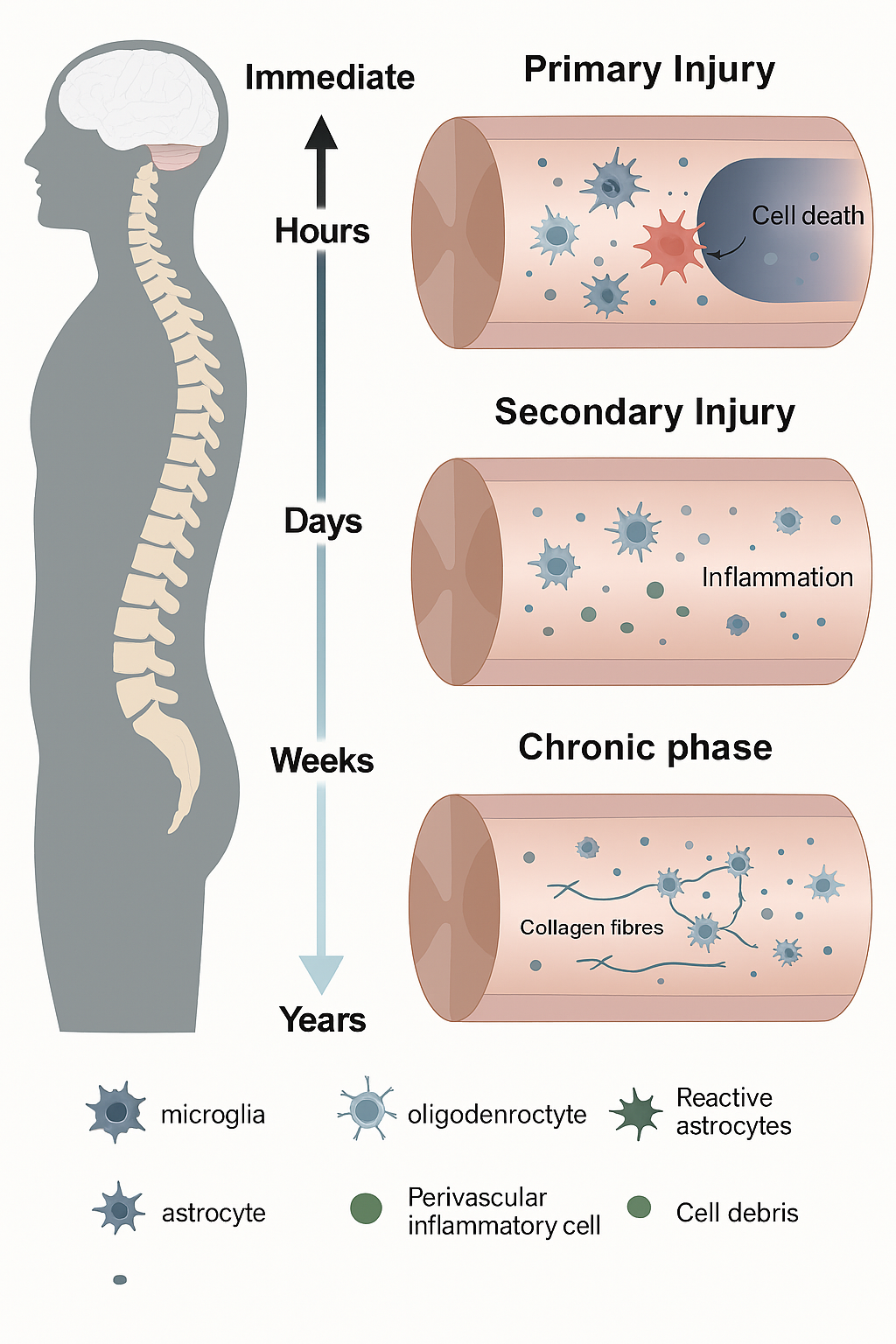
Spinal cord injury (SCI) is a catastrophic condition that leads to profound and often irreversible physical, neurological, and psychological impairments. Individuals affected by SCI face partial or complete loss of motor function, sensory perception, and autonomic control, which drastically diminishes quality of life and imposes substantial socioeconomic burdens (Smith & Taylor, 2015). Globally, the incidence of SCI is estimated to range from 250,000 to 500,000 cases annually, with trauma—such as vehicular accidents, falls, and sports injuries—being the primary cause (Smith et al., 2015). The complex pathophysiology of SCI involves an initial mechanical injury followed by a cascade of secondary processes including inflammation, oxidative stress, excitotoxicity, and apoptosis of neural cells, which further exacerbate tissue damage (Courtine & Sofroniew, 2019). These multifaceted challenges make SCI a condition with limited therapeutic options, as conventional treatments mainly focus on symptomatic management, rehabilitation, and supportive care rather than true neurological repair (Jones & Carter, 2016).
In recent years, attention has turned toward natural therapeutics, particularly adaptogenic fungi, which have been recognized for their potential to promote neuroprotection and tissue regeneration. Adaptogenic fungi, including Hericium erinaceus (Lion’s Mane), Cordyceps sinensis, Ganoderma lucidum (Reishi), and Inonotus obliquus (Chaga), have long been utilized in traditional medicine systems such as Traditional Chinese Medicine (TCM) and Ayurveda for their capacity to enhance resilience to physiological and psychological stress (Wang & Zhang, 2015; Xu et al., 2017). These fungi contain bioactive compounds, including polysaccharides, triterpenes, and hericenones, which exhibit anti-inflammatory, antioxidant, immunomodulatory, and neuroregenerative properties (Lee et al., 2017). Preclinical research suggests that such compounds may mitigate secondary injury processes following SCI, support neuronal repair, and enhance overall recovery outcomes (Patel, Singh, & Kumar, 2016).
Among these fungi, Hericium erinaceus has garnered significant attention due to its ability to stimulate nerve growth factor (NGF) synthesis, a key protein responsible for neuronal survival, differentiation, and axonal regeneration (Kawagishi, Zhuang, & Shimizu, 2014). NGF plays a pivotal role in repairing damaged neural circuits and promoting neuroplasticity, which is crucial for functional recovery after SCI. Preclinical studies demonstrate that supplementation with H. erinaceus can enhance NGF expression, promote axonal regrowth, and improve myelination in damaged spinal cord tissues, translating into measurable improvements in motor and cognitive functions (Li, Wang, & Zhang, 2015). Similarly, Cordyceps sinensis has demonstrated potent anti-inflammatory and antioxidant effects, reduced neuronal apoptosis and mitigated oxidative stress in injured spinal cord tissue (Zhou at al., 2013). This fungus has also been shown to upregulate brain-derived neurotrophic factor (BDNF), which supports synaptic plasticity and neural network reorganization, further facilitating functional recovery (Wang & Zhang, 2015).
Ganoderma lucidum (Reishi) offers additional benefits by modulating immune function and enhancing resistance to secondary infections, which are common complications in SCI patients (Lin & Zhang, 2015). Its bioactive polysaccharides and triterpenes regulate cytokine production, inhibit pro-inflammatory pathways, and improve antioxidant defenses, thereby reducing tissue damage and promoting a favorable environment for neural regeneration (Lee et al., 2017). Inonotus obliquus (Chaga), rich in polyphenols and melanins, complements these effects by providing robust antioxidant support, reducing oxidative stress, and maintaining gut barrier integrity, which indirectly contributes to improved systemic immunity and neurological outcomes (Patel et al., 2016; Lee et al., 2017). Together, these fungi offer a multifaceted approach to SCI management, addressing both the primary neuronal damage and the secondary pathological processes that hinder recovery.
A particularly emerging area of interest is the modulation of the gut-brain axis by adaptogenic fungi. SCI often leads to autonomic dysfunction, resulting in gastrointestinal dysbiosis, which can exacerbate neuroinflammation and compromise immune resilience (Patel et al., 2016). Compounds in H. erinaceus and C. sinensis have prebiotic properties, supporting the growth of beneficial gut microbiota and thereby promoting a balanced immune response and reduced systemic inflammation (Wang & Zhang, 2015). By enhancing gut health, these fungi indirectly influence neural repair mechanisms, illustrating a complex and integrated therapeutic potential that extends beyond the spinal cord itself.
While preclinical studies consistently indicate promising outcomes, clinical research remains limited. Small-scale human studies have reported improvements in motor function, cognitive performance, fatigue management, and overall well-being in SCI patients supplemented with these fungi, though effect sizes remain modest and variable (Lin & Zhang, 2015; Wang & Zhang, 2015). The lack of standardized extract formulations, optimal dosing regimens, and long-term safety data poses significant barriers to widespread clinical application. Moreover, the interaction between fungal supplements and conventional SCI therapies, such as pharmacological agents, physical rehabilitation, and stem cell interventions, remains underexplored (Jones & Carter, 2016).
Given the multidimensional challenges faced by SCI patients and the limitations of current treatment modalities, adaptogenic fungi present a promising complementary approach that may enhance neuroprotection, functional recovery, and quality of life. Understanding their bioactive mechanisms, clinical efficacy, and safety profiles is essential to bridging traditional medicine with modern neurological rehabilitation. This review aims to synthesize current scientific evidence on the role of adaptogenic fungi in SCI, highlighting their neuroprotective, anti-inflammatory, and regenerative properties, while discussing practical challenges and future research directions necessary for their integration into mainstream clinical practice. By leveraging both traditional knowledge and modern scientific research, adaptogenic fungi have the potential to reshape therapeutic strategies for spinal cord injury.
This review aims to critically evaluate the therapeutic potential of adaptogenic fungi in spinal cord injury management. The objectives are to examine their neuroprotective, anti-inflammatory, and regenerative properties, explore mechanisms such as nerve growth factor stimulation and oxidative stress reduction, and identify gaps in clinical research to guide future studies and clinical applications.