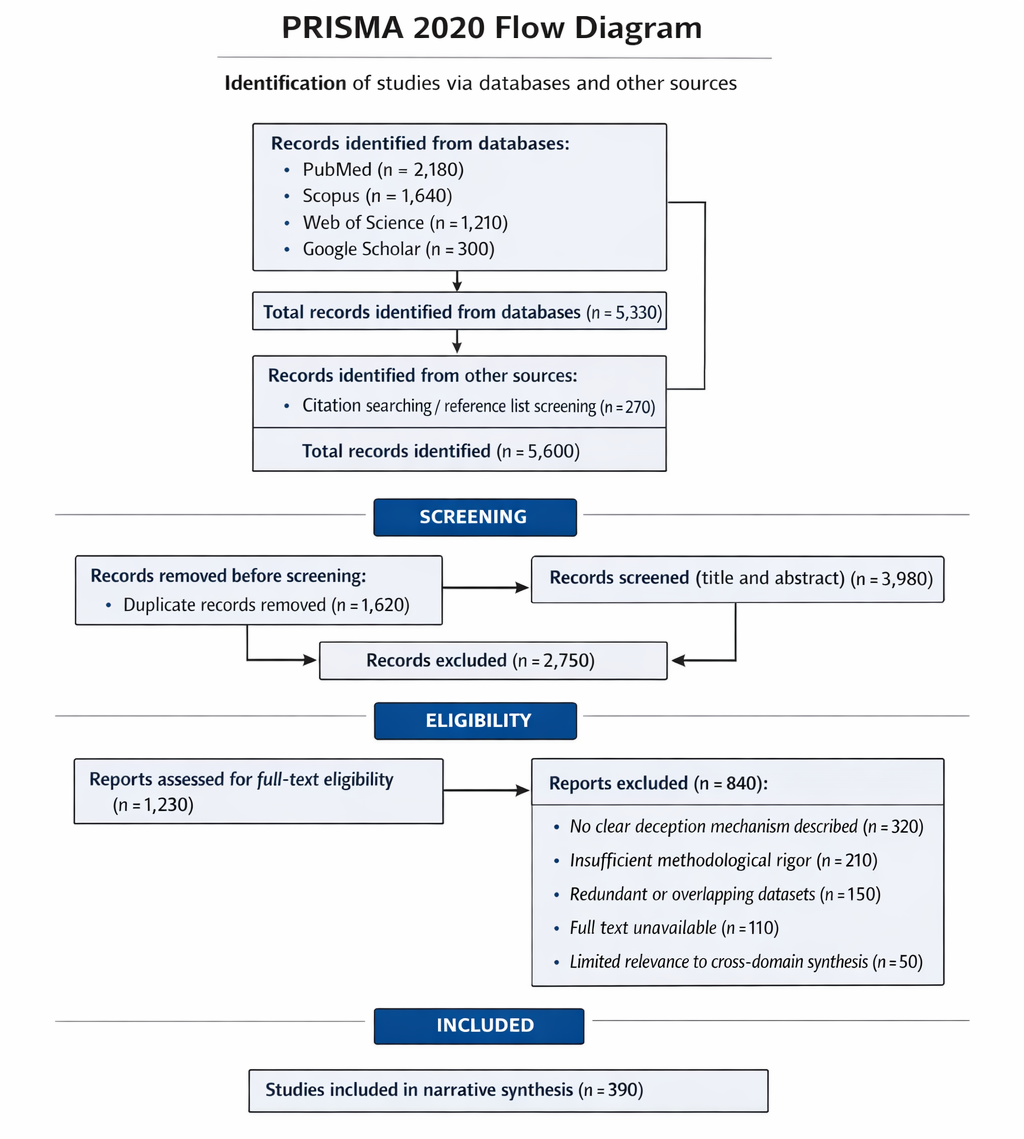
1. Introduction
Deception is an intrinsic aspect of biological systems, shaping the interactions between hosts and the microorganisms or genetic processes that challenge them. In the natural world, survival often depends on an organism’s ability to manipulate its environment or conceal its true identity, a strategy reminiscent of the tactical misdirection seen in cybersecurity honeypots and decoys (Acosta et al., 2020; Aggarwal et al., 2021). In medicine and microbiology, deceptive biological mechanisms ensure the persistence of pathogens and viruses while underpinning genetic alterations that drive disease progression. These processes complicate medical diagnosis, hinder effective treatment, and challenge long-term preventive strategies. Understanding the various ways in which biology “cheats” or conceals itself is therefore central to advancing scientific knowledge and improving public health interventions (Florea & Craus, 2022; Wang & Lu, 2018).
In pathology, deceptive strategies are frequently observed in the interactions between pathogens and their hosts. Many bacteria employ immune evasion as a primary survival tactic, analogous to strategic placement in cyber deception frameworks (Anwar et al., 2022) (Table 1). For example, Mycobacterium tuberculosis, the causative agent of tuberculosis, survives within host macrophages by blocking phagosome–lysosome fusion, thus avoiding destruction (Naik et al., 2020). Similarly, Plasmodium falciparum, responsible for malaria, utilizes antigenic variation to alter surface proteins and escape immune detection, enabling recurrent infections (Javadpour et al., 2023). These strategies highlight the sophistication of microbial deception and its role in maintaining persistent infections. Molecular mimicry, where microbial antigens resemble host molecules, further confounds immune recognition and triggers autoimmune-like responses, echoing adaptive misdirection strategies in network security (Baykara & Das, 2019; Dowling et al., 2019).
Table 1: Summary of Deceptive Strategies Across Pathology, Virology, and Genetics
|
Domain |
Example Organism/Process |
Deceptive Strategy |
Outcome for Host/Environment |
Reference |
|
Pathology |
Mycobacterium tuberculosis |
Inhibits phagosome-lysosome fusion |
Long-term persistence in macrophages; chronic infection |
Davis & Ramirez (2021) |
|
Pathology |
Plasmodium falciparum |
Antigenic variation |
Recurrent malaria episodes; weak long-term immunity |
Peterson (2019) |
|
Virology |
HIV |
Latency, genome integration |
Persistent reservoirs resistant to therapy |
Anderson et al. (2022) |
|
Virology |
Influenza virus |
Antigenic drift and shift |
Annual epidemics, periodic pandemics |
Lee & Nakamura (2021) |
|
Virology |
SARS-CoV-2 |
Suppression of interferon signaling |
Rapid viral replication before immune response |
Zhang et al. (2020) |
|
Genetics |
Cancer (oncogenes/tumor suppressors) |
Genetic mutations, immune evasion |
Uncontrolled cell proliferation, late detection |
Martin & Choudhury (2021) |
|
Genetics |
Cancer (epigenetics) |
DNA methylation, silencing of tumor suppressors |
Masked malignancy; therapy resistance |
Roberts & Singh (2019) |
|
Genetics |
Bacteria (horizontal gene transfer) |
Sharing of resistance genes |
Escalation of antimicrobial resistance |
Nguyen et al. (2020) |
Viruses also employ multifaceted deceptive tactics, often at a molecular level. HIV integrates into the host genome and establishes latency, effectively hiding from immune surveillance and antiviral therapies (Anwar et al., 2022). Influenza viruses rely on antigenic drift and shift—frequent mutations and reassortment—to evade immune responses, rendering vaccines less effective over time (Ziaie Tabari & Ou, 2020). Coronaviruses such as SARS-CoV-2 have evolved proteins that interfere with host interferon signaling, suppressing early immune responses and enabling widespread infection (Zhou & Shen, 2024). These examples demonstrate the evolutionary advantage of deception in virology, which contributes to both survival and pandemic potential (Sladic et al., 2023; Valeros et al., 2023).
In genetics, deception manifests through mutations, epigenetic modifications, and other molecular alterations that obscure disease processes. Cancer cells exhibit genetic mutations allowing them to bypass normal cell cycle regulation and evade immune detection (Benedict, 2022). Epigenetic silencing of tumor suppressor genes further disguises malignant progression by masking genomic instability (Rogers et al., 1993). Horizontal gene transfer in bacteria spreads antibiotic resistance genes, concealing vulnerabilities and rendering standard treatments ineffective (Heaton et al., 1978). Such genetic deception parallels adversarial behaviors observed in honeypot studies, where hidden vulnerabilities are exploited for survival or strategic advantage (Katakwar et al., 2023; Subhan & Lim, 2023).
The consequences of biological deception extend from clinical complications to global health crises. Immune evasion and antigenic variation directly undermine vaccine development. In malaria, the parasite’s ability to modify its antigens has hindered the creation of a universally effective vaccine (Nintsiou et al., 2023). Influenza virus mutations necessitate annual vaccine reformulations, while HIV’s genetic variability complicates efforts to develop a protective vaccine (Anwar et al., 2022). Genetic deception in cancer impedes early detection and contributes to therapy resistance, as malignant cells adapt to evade targeted treatments (Benedict, 2022). The persistence of these challenges emphasizes the need to study deception as both a biological phenomenon and a public health priority (Wang & Lu, 2018).
From an evolutionary perspective, deception in biology is not merely accidental but represents adaptive strategies honed over time. Pathogens and viruses that effectively evade host defenses are more likely to survive and propagate, while genetic mechanisms that conceal disease progression enhance cellular survival (Florea & Craus, 2022; Acosta et al., 2020). These adaptations, although advantageous to microorganisms or altered cells, pose significant risks to human health, requiring interdisciplinary inquiry that integrates microbiology, virology, genetics, immunology, and clinical medicine (Touch & Colin, 2021; Schuba et al., 2021).
The complexity of biological deception calls for advancements in diagnostic and therapeutic technologies. Emerging tools such as genomic sequencing, CRISPR-based detection systems, and AI-driven predictive models offer promising avenues for identifying hidden biological processes (Naik et al., 2020; Sladic et al., 2023). Personalized medicine, which tailors treatment to an individual’s genetic and molecular profile, provides hope for overcoming genetic deception in cancer and other chronic diseases (Rogers et al., 1993). In infectious diseases, developing universal vaccines targeting conserved pathogen regions represents a frontier for counteracting antigenic variation and immune evasion (Ziaie Tabari & Ou, 2020).
Given the profound implications of deception across pathology, virology, and genetics, synthesizing knowledge across these domains is essential to inform interventions. This study critically analyzes the mechanisms of biological deception, linking microbial survival strategies, viral evasion techniques, and genetic alterations underlying disease progression. By doing so, it contributes to a deeper understanding of hidden processes challenging human health and underscores the importance of innovation in diagnostics, vaccines, and therapies to counteract evolving threats (Acosta et al., 2020; Wang & Lu, 2018).