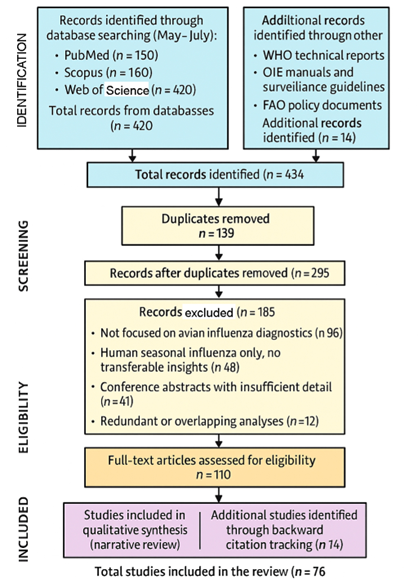
1. Introduction
Emerging infectious diseases remain one of the greatest challenges to global public health in the twenty-first century. Among these, avian influenza has repeatedly demonstrated its ability to disrupt animal health systems, damage economies, and threaten human wellbeing. Since the early outbreaks of highly pathogenic avian influenza (HPAI) H5N1 in the late 1990s, concerns about the potential for zoonotic transmission and even pandemic spread have been at the forefront of international health agendas (Capua & Alexander, 2007). The ability to detect cases quickly and accurately is therefore not simply a scientific necessity, but a societal imperative. Diagnostic tools act as the first line of defence in surveillance, guiding interventions, and building trust in the health response. However, when these tools are not adequately validated, the consequences can be far-reaching.
The term “validation” refers to the rigorous process by which a diagnostic test is assessed for its sensitivity, specificity, and reproducibility across different contexts (World Health Organization [WHO], 2018). Without this process, results can become unreliable, producing false positives that exaggerate the scale of an outbreak, or false negatives that miss circulating infections entirely (Spackman & Suarez, 2008). In either case, the implications are serious. Farmers may experience devastating losses from unnecessary culling, while communities may lose confidence in veterinary and public health institutions. Equally troubling, poorly validated diagnostics can distort epidemiological data, making it difficult to assess the true burden of disease or to allocate resources effectively (Swayne et al., 2020).
The misuse of non-validated diagnostic assays is not a purely technical problem; it is also rooted in broader human and institutional behaviours. In some cases, laboratories under pressure to deliver quick results adopt assays that have not been peer-reviewed or standardised. In others, resource-limited settings may rely on cheaper, less reliable alternatives because validated molecular methods such as reverse transcription polymerase chain reaction (RT-PCR) demand advanced infrastructure and skilled personnel (Alexander, 2009). These choices, while understandable in context, raise critical questions about equity and responsibility. If wealthier nations can afford state-of-the-art diagnostics while poorer ones rely on shortcuts, global surveillance becomes fragmented, and collective security is weakened (OIE, 2015).
At the heart of the issue lies a tension between speed and reliability. During outbreaks, governments and health authorities are often under immense pressure to produce immediate answers. Non-validated assays can appear attractive because they are faster to deploy or easier to interpret. Yet, the long-term costs of inaccuracy almost always outweigh the short-term gains. For example, during past HPAI outbreaks in Asia, reliance on rapid antigen tests with poor sensitivity led to underestimation of disease prevalence, which delayed control measures and allowed further spread (Chen et al., 2010). Conversely, false alarms have resulted in mass poultry culling, disrupting food supply chains and livelihoods, while also eroding public trust when subsequent investigations revealed no evidence of infection (Van Kerkhove, 2013).
Validated molecular methods, particularly RT-PCR, have been consistently demonstrated as the gold standard for avian influenza detection. These assays target conserved regions of the viral genome, offering both high sensitivity and specificity (Spackman et al., 2002). Beyond their technical reliability, validated tools also contribute to global harmonisation of data. When laboratories across different regions adopt standard protocols, results become comparable, facilitating coordinated responses to transboundary threats (WHO, 2018). However, the benefits of these technologies can only be fully realised if they are accompanied by investment in training, quality control systems, and inter-laboratory proficiency testing.
The implications of using non-validated tools extend beyond immediate outbreak management. At a deeper level, they touch on issues of scientific credibility and public trust. In an era marked by widespread misinformation and scepticism toward expert authority, the revelation that diagnostic results may have been produced by unreliable methods risks fuelling distrust not only in health systems but also in the science underpinning them (Larson, 2018). Public confidence is not an abstract concern—it directly influences compliance with control measures, from vaccination campaigns to biosecurity regulations.
This study therefore takes a critical look at the misuse of non-validated diagnostic assays in the context of avian influenza and related unidentified or non-pathogenic molecules. It highlights the dangers of relying on tools that lack proper validation, not only in technical terms but also in relation to their broader social, economic, and political consequences. While advances in molecular biology have given us increasingly powerful diagnostic options, these advances also bring responsibilities: to validate carefully, to standardise internationally, and to communicate transparently.
The aim of this paper is to critically examine the implications of using non-validated diagnostic tools for avian influenza and related unidentified molecules, with particular focus on their impact on surveillance accuracy, outbreak management, and public trust. To achieve this, the study pursues three key objectives: first, to analyse the limitations and risks associated with non-validated assays in terms of diagnostic reliability and epidemiological consequences; second, to evaluate the strengths of validated molecular diagnostics, particularly reverse transcription polymerase chain reaction (RT-PCR), in improving sensitivity, specificity, and global comparability; and third, to highlight the broader social, economic, and scientific implications of diagnostic misuse, thereby underscoring the need for international standardisation, capacity-building, and adherence to quality control frameworks.