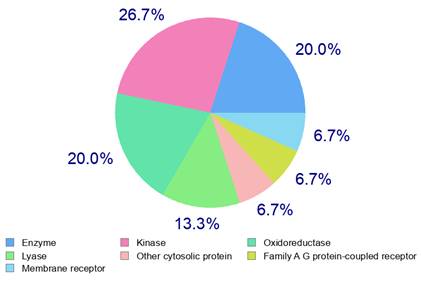
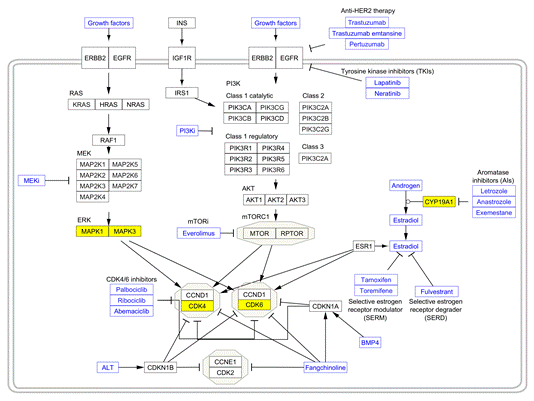
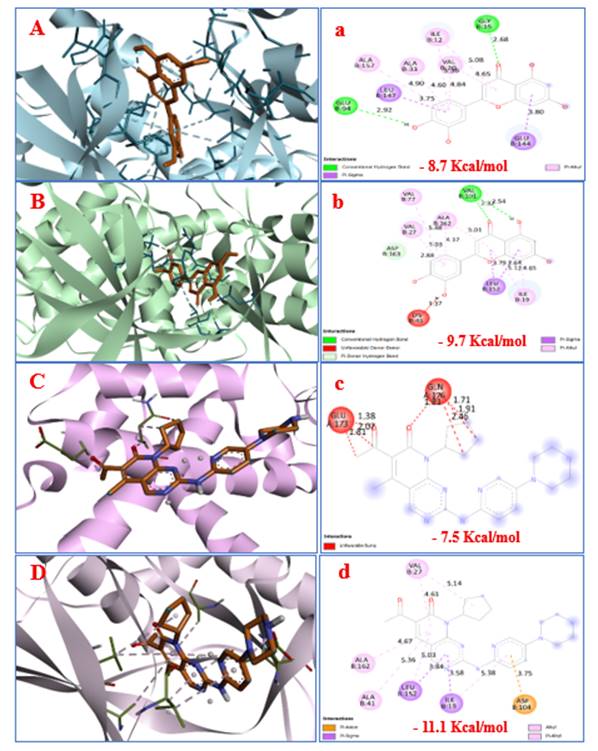
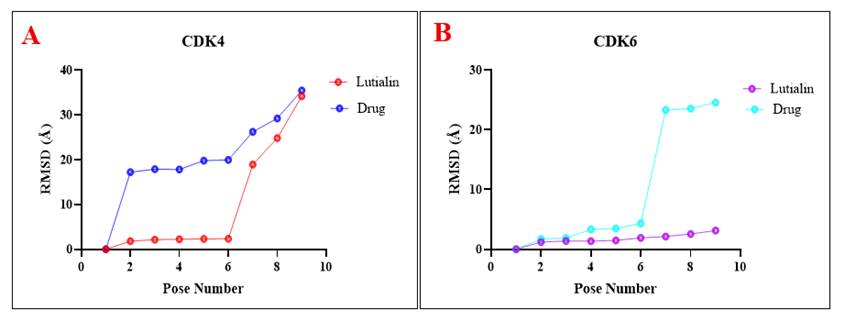
Akter, M. F., Islam, M. R., Manica, A. K., Siddique, M. A. B., Tufael (2022). "Structural and Pharmacological Insights into Withaferin a Binding to Mutant p53 (R248Q): Multi-Faceted Inhibitor in Cancer Treatment", Journal of Angiotherapy, 6(2),1-8,10432. https://doi.org/10.25163/angiotherapy.6210432
Akter, M. F., Manica, A. K., Siddique, M. A. B., Tufael, Islam, M. R., Nusrat, S. (2025). "Structural Insights into TEM-1 β-Lactamase-Mediated Ceftriaxone Resistance in Escherichia coli: A Molecular Docking and Toxicity Analysis", Microbial Bioactives, 8(1), 1-11, 10447 https://doi.org/10.25163/microbbioacts.8110447
Amin, M. S., Rashid, M. J., Tufael, Rahman, A. (2025). "Probiotics as Emerging Neurotherapeutics in Spinal Cord Injury: Modulating Inflammation, Infection, and Regeneration", Microbial Bioactives, 8(1), 1-11, 10290 https://doi.org/10.25163/microbbioacts.8110290
Bizuayehu, H. M., Ahmed, K. Y., Kibret, G. D., Dadi, A. F., Belachew, S. A., Bagade, T., Tegegne, T. K., Venchiarutti, R. L., Kibret, K. T., Hailegebireal, A. H., Assefa, Y., Khan, M. N., Abajobir, A., Alene, K. A., Mengesha, Z., Erku, D., Enquobahrie, D. A., Minas, T. Z., Misgan, E., & Ross, A. G. (2024). Global Disparities of Cancer and Its Projected Burden in 2050. JAMA Network Open, 7(11), e2443198. https://doi.org/10.1001/jamanetworkopen.2024.43198
EL Omri, A., Han, J., Kawada, K., Ben Abdrabbah, M., & Isoda, H. (2012). Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Research, 1437, 16–25. https://doi.org/10.1016/j.brainres.2011.12.019
Gao, H.-L., Yu, X.-J., Feng, Y.-Q., Yang, Y., Hu, H.-B., Zhao, Y.-Y., Zhang, J.-H., Liu, K.-L., Zhang, Y., Fu, L.-Y., Li, Y., Qi, J., Qiao, J.-A., & Kang, Y.-M. (2023). Luteolin Attenuates Hypertension via Inhibiting NF-κB-Mediated Inflammation and PI3K/Akt Signaling Pathway in the Hypothalamic Paraventricular Nucleus. Nutrients, 15(3), 502. https://doi.org/10.3390/nu15030502
Goel, S., Bergholz, J. S., & Zhao, J. J. (2022). Targeting CDK4 and CDK6 in cancer. Nature Reviews Cancer, 22(6), 356–372. https://doi.org/10.1038/s41568-022-00456-3
Guo, X., Yu, X., Zheng, B., Zhang, L., Zhang, F., Zhang, Y., Li, J., Pu, G., Zhang, L., & Wu, H. (2021). Network Pharmacology-Based Identification of Potential Targets of Lonicerae japonicae Flos Acting on Anti-Inflammatory Effects. BioMed Research International, 2021(1). https://doi.org/10.1155/2021/5507003
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schiöth, H. B., & Gloriam, D. E. (2017). Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery, 16(12), 829–842. https://doi.org/10.1038/nrd.2017.178
Hu, Y., Gao, J., Wang, M., & Li, M. (2021). Potential Prospect of CDK4/6 Inhibitors in Triple-Negative Breast Cancer. Cancer Management and Research, Volume 13, 5223–5237. https://doi.org/10.2147/CMAR.S310649
Huang, A., Fang, G., Pang, Y., & Pang, Z. (2019). A Network Pharmacology Approach to Explore Mechanism of Action of Longzuan Tongbi Formula on Rheumatoid Arthritis. Evidence-Based Complementary and Alternative Medicine, 2019, 1–13. https://doi.org/10.1155/2019/5191362
Islam, M. R., Manica, A. K., Akter, M. F., Siddique, M. A. B., Tufael (2023). "In Silico Drug-Likeness and Safety Profiling of Tinosporaside: A Natural Alternative to Celecoxib for COX-2 Inhibition", Journal of Primeasia, 4(1),1-8,10434. https://doi.org/10.25163/primeasia.4110434
Li, Q., Wang, S., Wu, Z., & Liu, Y. (2020). DDX11-AS1exacerbates bladder cancer progression by enhancing CDK6 expression via suppressing miR-499b-5p. Biomedicine & Pharmacotherapy, 127, 110164. https://doi.org/10.1016/j.biopha.2020.110164
Li, X., Niu, C., Yi, G., Zhang, Y., Jin, W., Zhang, Z., Zhang, W., & Li, B. (2024). Quercetin inhibits the epithelial-mesenchymal transition and reverses CDK4/6 inhibitor resistance in breast cancer by regulating circHIAT1/miR-19a-3p/CADM2 axis. PLOS ONE, 19(7), e0305612. https://doi.org/10.1371/journal.pone.0305612
Lopez-Tarruella, S., Echavarria, I., Jerez, Y., Herrero, B., Gamez, S., & Martin, M. (2022). How we Treat HR-Positive, HER2-Negative Early Breast Cancer. Future Oncology, 18(8), 1003–1022. https://doi.org/10.2217/fon-2021-0668
Merecz-Sadowska, A., Sadowski, A., Zielinska-Blizniewska, H., Zajdel, K., & Zajdel, R. (2025). Network Pharmacology as a Tool to Investigate the Antioxidant and Anti-Inflammatory Potential of Plant Secondary Metabolites—A Review and Perspectives. International Journal of Molecular Sciences, 26(14), 6678. https://doi.org/10.3390/ijms26146678
Manica, A. K., Akter, M. F., Islam, M. R., Tufael, Siddique, M. A. B. (2022). "Computational Exploration of Xanthohumol as a Safer Natural Substitute for Tamoxifen in Estrogen Receptor-Positive Breast Cancer", Journal of Angiotherapy, 6(2),1-8,10431. https://doi.org/10.25163/angiotherapy.6210431
Manica, A. K., Tufael, Siddique, M. A. B., Akter, M. F., Islam, M. R. (2023). "In Silico Repurposing of FDA-approved Drugs Targeting Keap1-NRF2 Axis in Hepatocellular Carcinoma for Precision Therapy", Journal of Precision Biosciences, 5(1),1-8,10436. https://doi.org/10.25163/biosciences.5110436
Manica, A. K., Siddique, M. A. B., Tufael, Akter, M. F., Islam, M. R. (2024). "Targeted Drug Repurposing in Precision Oncology Reveals Celecoxib as a GSK-3β Inhibitor in Hepatocellular Carcinoma", Journal of Precision Biosciences, 6(1),1-8,10440. https://doi.org/10.25163/biosciences.6110440
Manica, A. K., Islam, M. R., Siddique, M. A. B., Akter, M. F., Tufael (2024). "Tanshinone IIA as a Promising Natural Inhibitor of the STING Pathway: A Computational Exploration Toward Neuroinflammatory Therapy", Australian Herbal Insight, 7(1),1-8,10441. https://doi.org/10.25163/herbal.7110441
Moradifar, F., Sepahdoost, N., Tavakoli, P., & Mirzapoor, A. (2025). Multi-functional dressings for recovery and screenable treatment of wounds: A review. Heliyon, 11(1), e41465. https://doi.org/10.1016/j.heliyon.2024.e41465
Nardone, V., Barbarino, M., Angrisani, A., Correale, P., Pastina, P., Cappabianca, S., Reginelli, A., Mutti, L., Miracco, C., Giannicola, R., Giordano, A., & Pirtoli, L. (2021). CDK4, CDK6/cyclin-D1 Complex Inhibition and Radiotherapy for Cancer Control: A Role for Autophagy. International Journal of Molecular Sciences, 22(16), 8391. https://doi.org/10.3390/ijms22168391
Rahman, S. S., Klamrak, A., Mahat, N. C., Rahat, R. H., Nopkuesuk, N., Kamruzzaman, M., Janpan, P., Saengkun, Y., Nabnueangsap, J., Soonkum, T., Sangkudruea, P., Jangpromma, N., Kulchat, S., Patramanon, R., Chaveerach, A., Daduang, J., & Daduang, S. (2025). Thyroid Stimulatory Activity of Houttuynia cordata Thunb. Ethanolic Extract in 6-Propyl-Thiouracil-Induced Hypothyroid and STZ Induced Diabetes Rats: In Vivo and In Silico Studies. Nutrients, 17(3), 594. https://doi.org/10.3390/nu17030594
Ran, R., Ma, Y., Wang, H., Yang, J., & Yang, J. (2022). Treatment strategies for hormone receptor-positive, human epidermal growth factor receptor 2-positive (HR+/HER2+) metastatic breast cancer: A review. Frontiers in Oncology, 12. https://doi.org/10.3389/fonc.2022.975463
Roufayel, R., Mezher, R., & Storey, K. B. (2021). The Role of Retinoblastoma Protein in Cell Cycle Regulation: An Updated Review. Current Molecular Medicine, 21(8), 620–629. https://doi.org/10.2174/1566524020666210104113003
Sager, R. A., Backe, S. J., Ahanin, E., Smith, G., Nsouli, I., Woodford, M. R., Bratslavsky, G., Bourboulia, D., & Mollapour, M. (2022). Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma. Nature Reviews Urology, 19(5), 305–320. https://doi.org/10.1038/s41585-022-00571-8
Singh Tuli, H., Rath, P., Chauhan, A., Sak, K., Aggarwal, D., Choudhary, R., Sharma, U., Vashishth, K., Sharma, S., Kumar, M., Yadav, V., Singh, T., Yerer, M. B., & Haque, S. (2022). Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers, 14(21), 5373. https://doi.org/10.3390/cancers14215373
Siddique, M. A. B., Debnath, A., Ullah, M. S., Amin, M. S., Rahman, A., Mou, M. A., Biswash, M. A. R., Tamim, M. S. B. N., Akter, M. S., Ahmed, B., Numan, A. A., Shabuj, M. M. H. (2025). "Targeting p38 MAPK: Molecular Docking and Therapeutic Insights for Alzheimer’s Disease Management", Journal of Primeasia, 6(1),1-11,10116. https://doi.org/10.25163/primeasia.6110116
Sun, C., Jiang, C., Wang, X., Ma, S., Zhang, D., & Jia, W. (2024). MR-based radiomics predicts CDK6 expression and prognostic value in high-grade glioma. Academic Radiology, 31(12), 5141-5153. https://doi.org/10.1016/j.acra.2024.06.006
Tadesse, S., Yu, M., Kumarasiri, M., Le, B. T., & Wang, S. (2015). Targeting CDK6 in cancer: State of the art and new insights. Cell Cycle, 14(20), 3220–3230. https://doi.org/10.1080/15384101.2015.1084445
Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., & Dzobo, K. (2018). Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. International Journal of Molecular Sciences, 19(6), 1578. https://doi.org/10.3390/ijms19061578
Tufael, Debnath, A., Siddique, M. A. B., Nath, N. D. (2023). "Microbial Therapeutics in Cancer Treatment - Challenges and Opportunities in Breast Cancer Management", Clinical Epidemiology & Public Health, 1(1),1-7,10277. https://doi.org/10.25163/health.1110277
Tufael, Md Mostafizur Rahman et al. (2024). Combined Biomarkers for Early Diagnosis of Hepatocellular Carcinoma, Journal of Angiotherapy, 8(5), 1-12, 9665. https://doi.org/10.25163/angiotherapy.859665
Tufael, Kar, A., Rashid, M. H. O., Sunny, A. R., Raposo, A., Islam, M. S., Hussain, M. A., Hussen, M. A., Han, H., Coutinho, H. D. M., Ullah, M. S., & Rahman, M. M. (2024). Diagnostic efficacy of tumor biomarkers AFP, CA19-9, and CEA in Hepatocellular carcinoma patients. Journal of Angiotherapy, 8(4), Article 9513. https://doi.org/10.25163/angiotherapy.849513
Tufael, Akter, M. F., Islam, M. R., Siddique, M. A. B., Hassan, N., Numan, A. A., Naher, A. A., Shabuj, M. M. H., Manica, A. K., Shaikat, B., Rabbani, T. B. (2025). "Encoded Resistance: Structural Disruption and Signaling Crosstalk Undermine Sorafenib Binding in Mutant VEGFR2-Driven HCC", Paradise, 1(1),1-9,10427. https://doi.org/10.25163/paradise.1110427
Wang, W., Yang, C., Xia, J., Li, N., & Xiong, W. (2023). Luteolin is a potential inhibitor of COVID-19: An in silico analysis. Medicine, 102(38), e35029. https://doi.org/10.1097/MD.0000000000035029
Wang, X., Zhang, L., Dai, Q., Si, H., Zhang, L., Eltom, S. E., & Si, H. (2021). Combined Luteolin and Indole-3-Carbinol Synergistically Constrains ERα-Positive Breast Cancer by Dual Inhibiting Estrogen Receptor Alpha and Cyclin-Dependent Kinase 4/6 Pathway in Cultured Cells and Xenograft Mice. Cancers, 13(9), 2116. https://doi.org/10.3390/cancers13092116
Yan, J., Zhang, G., Pan, J., & Wang, Y. (2014). α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. International Journal of Biological Macromolecules, 64, 213–223. https://doi.org/10.1016/j.ijbiomac.2013.12.007
Zhao, X., Yu, X., Li, W., Chen, Z., Niu, T., Weng, X., Wang, L., & Liu, X. (2024). CDK6 as a Biomarker for Immunotherapy, Drug Sensitivity, and Prognosis in Bladder Cancer: Bioinformatics and Immunohistochemical Analysis. International Journal of Medical Sciences, 21(12), 2414–2429. https://doi.org/10.7150/ijms.101043