1. Introduction
The human gut microbiota, a complex and dynamic ecosystem comprising trillions of microorganisms, plays a pivotal role in maintaining host health through metabolic, immune, and neurobiological pathways (Belkaid & Hand, 2014; Ghosh et al., 2020). This diverse microbial community influences essential physiological processes, including nutrient metabolism, immune homeostasis, and protection against pathogens (Shreiner et al., 2015). Over the past two decades, research has revealed that disturbances in the gut microbiome—referred to as dysbiosis—are associated with a wide range of diseases, including metabolic disorders, inflammatory bowel disease (IBD), neurodegenerative diseases, and psychiatric conditions (Kostic et al., 2014; Dinan & Cryan, 2017; Cheng et al., 2019). Consequently, modulating the gut microbiota through probiotics, prebiotics, and postbiotics has emerged as a promising therapeutic strategy to restore microbial balance and enhance human health (Cunningham et al., 2021).
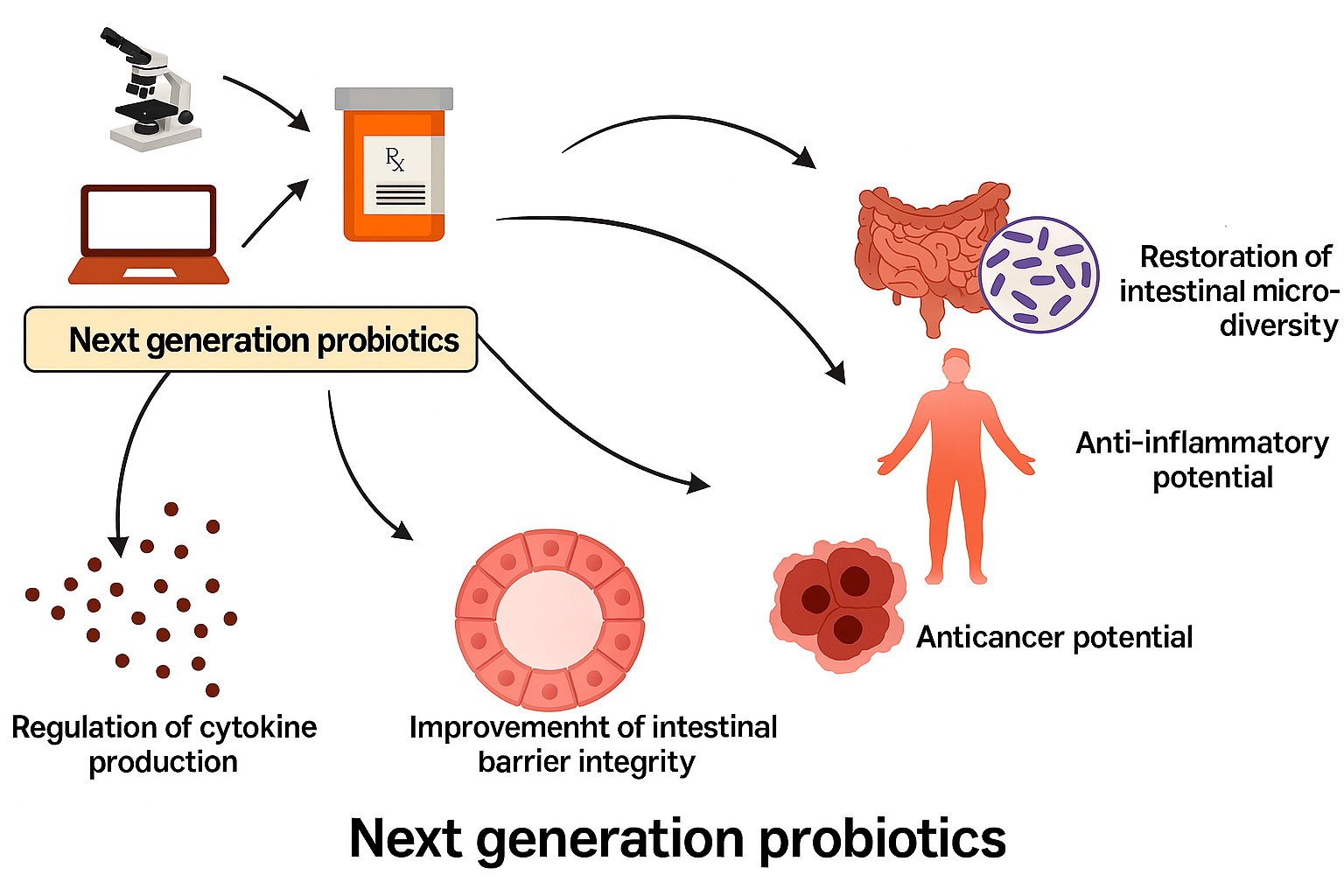
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer health benefits to the host (Suez et al., 2018). Traditionally, probiotics such as Lactobacillus and Bifidobacterium species have been employed to maintain intestinal health and improve digestion. However, advances in sequencing technologies and metagenomics have expanded the understanding of probiotic function, leading to the discovery of next-generation probiotics with targeted therapeutic potential (Abouelela & Helmy, 2024). Notably, Akkermansia muciniphila, a mucin-degrading bacterium, has demonstrated metabolic and immunomodulatory benefits, with clinical trials showing its ability to improve insulin sensitivity and reduce inflammation in overweight and obese individuals (Depommier et al., 2019). Such findings underscore the growing potential of specific bacterial strains in disease prevention and management beyond gastrointestinal health.
The gut microbiome also interacts intricately with the host immune system. It shapes immune cell differentiation, promotes tolerance to commensal organisms, and regulates inflammatory responses (Belkaid & Hand, 2014; Cani et al., 2019). Perturbations in these interactions can trigger chronic inflammatory conditions such as IBD, rheumatoid arthritis, and metabolic syndrome (Vaghef-Mehrabany et al., 2014; Pascale et al., 2018). For instance, Bacteroides-derived sphingolipids have been shown to maintain intestinal immune balance, preventing excessive inflammatory responses that contribute to intestinal disorders (Brown et al., 2019). Therefore, probiotics that modulate immune pathways are increasingly being studied as adjunct therapies in autoimmune and inflammatory diseases.
Another critical area of research is the gut-brain axis—a bidirectional communication network linking the enteric nervous system and the central nervous system through neural, hormonal, and immunological pathways (Mayer et al., 2014; Dinan & Cryan, 2017). The discovery that gut microbes can influence brain chemistry and emotional behavior has led to the rise of the term “psychobiotics,” describing probiotic strains capable of producing neuroactive substances such as gamma-aminobutyric acid (GABA), serotonin, and dopamine (Bravo et al., 2011; Sarkar et al., 2018). Experimental and clinical studies have demonstrated that probiotic administration can alleviate anxiety, depression, and cognitive decline, suggesting therapeutic applications for mental health and neurodegenerative disorders (Aarts et al., 2017; Kim et al., 2021; Jiang et al., 2017). For example, the ingestion of Lactobacillus rhamnosus in mice was found to regulate emotional behavior via vagus nerve signaling (Bravo et al., 2011), highlighting the potential for microbial-based interventions in neurological disorders.
Dietary factors exert a profound influence on the composition and functionality of the gut microbiota. Diverse and fiber-rich diets support microbial diversity, which is critical for resilience and disease resistance (Heiman & Greenway, 2016; Singh et al., 2021). Conversely, Western diets high in fat and sugar promote dysbiosis, leading to inflammation and metabolic disturbances (Koeth et al., 2013; Sommer et al., 2017). Personalized nutrition, guided by individual microbiome profiling, has therefore become a frontier in preventive healthcare (Johnson et al., 2020). By integrating dietary interventions with probiotic supplementation, personalized microbial modulation could become a key strategy for optimizing metabolic and mental health outcomes. Despite the growing enthusiasm, probiotic efficacy varies significantly across individuals due to host-specific factors such as genetics, baseline microbiota composition, and mucosal colonization resistance (Zmora et al., 2018). Studies have shown that not all individuals respond equally to the same probiotic strain, highlighting the need for precision-based approaches (Suez et al., 2018). Moreover, while probiotics are generally regarded as safe, challenges remain in standardizing strains, ensuring viability through gastrointestinal transit, and understanding their long-term effects (Cunningham et al., 2021). Emerging research is therefore focusing on engineered probiotics and postbiotics—non-viable microbial components and metabolites that can confer similar benefits without the challenges of live organism delivery (Abouelela & Helmy, 2024).