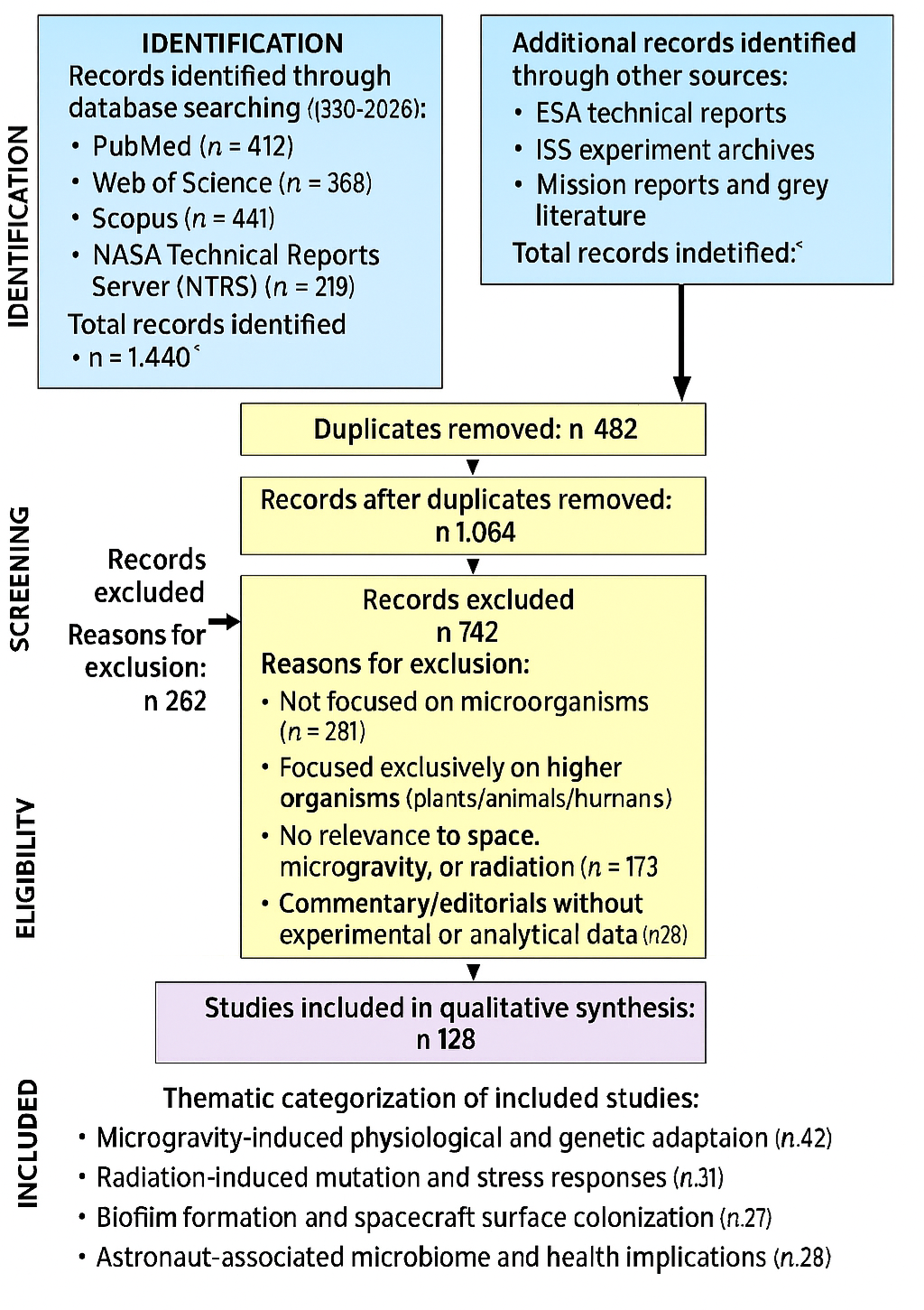
1. Introduction
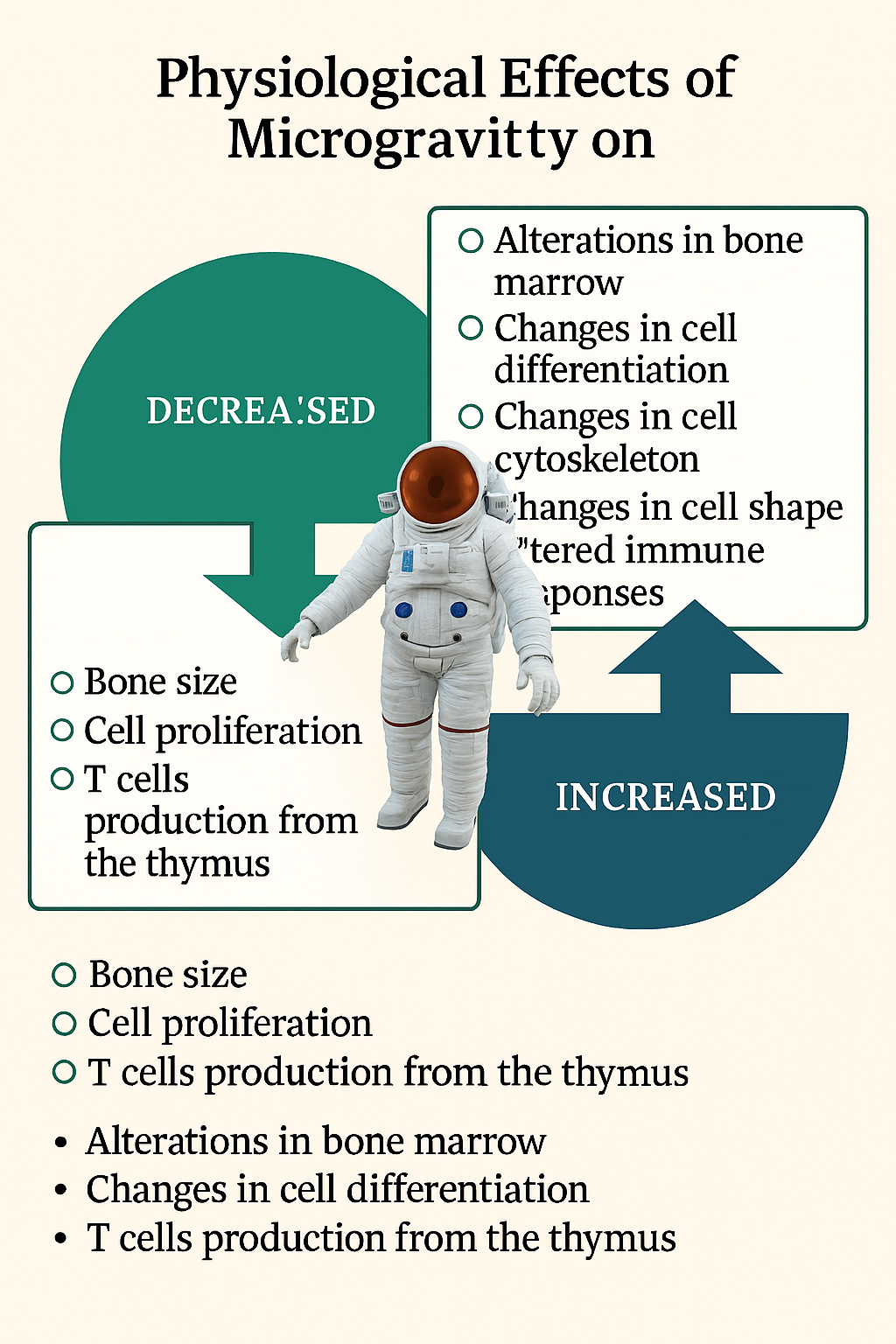
On Earth, microbial life has been observed to thrive in a variety of hostile conditions. It is estimated that microbial life may have evolved in the Archaean era (approximately 2,500 million years ago), even though Antonie van Leeuwenhoek first discovered microorganisms, or “animalcules,” in 1675. Microbes have since proven capable of enduring some of the most extreme environments known to humankind. As noted by Cavalier-Smith, Brasier, and Embley (2006), microorganisms have shaped Earth's biosphere through their long evolutionary history, fundamentally influencing biogeochemical cycles and the planet’s ecological stability. Biomarkers, biogenic isotope ratios, and microfossil evidence all provide insight into the origin and evolution of microbial life on Earth. Such biomarkers include the presence of ancient organic compounds that indicate metabolic activity dating back billions of years. The discovery of Archaea as a third domain of life by Carl Woese and colleagues revolutionized microbiology, evolutionary biology, and comparative genomics, highlighting the immense adaptability and diversity of microorganisms (Cavalier-Smith et al., 2006). Among the most resilient life forms are extremophiles, microorganisms that thrive under conditions once thought to be uninhabitable—such as hot springs, salt flats, acidic lakes, deep-sea vents, and polar ice. Their ability to survive in extreme conditions involving radiation, pressure, salinity, or pH extremes has expanded our understanding of life’s boundaries (Litchfield & Gillevet, 2002). These microbes not only challenge traditional definitions of habitability but also serve as models for studying the potential of life beyond Earth. Their remarkable physiological and molecular adaptations—such as efficient DNA repair mechanisms, spore formation, and membrane stabilization—demonstrate how life can persist under multiple stressors, including those resembling extraterrestrial environments (Grimm et al., 2014). Scientists have long speculated on the possibility of terrestrial microbial life surviving in outer space or on other planetary bodies. Investigating the “limits of life” and the boundaries of microbial survival is critical for understanding the origins of life and its potential distribution across the universe. Space provides a unique natural laboratory for exploring these questions. The space environment exposes organisms to a combination of stressors including microgravity, intense cosmic and solar radiation, desiccation, and vacuum—all of which test the resilience of life in ways that terrestrial laboratories cannot fully replicate (Cockell et al., 2011; Deguchi et al., 2011). Early research into microbial survival in the upper atmosphere laid the groundwork for space microbiology. For instance, microorganisms were first collected from high altitudes in the 1930s, providing evidence that bacterial and fungal spores could persist in the stratosphere despite low pressure and radiation exposure (Griffin, 2004). These findings led to subsequent experiments using balloon- and rocket-borne sampling missions to examine microbial presence in the stratosphere and mesosphere (DeLeon-Rodriguez et al., 2013). The successful recovery of viable spores from such altitudes demonstrated that microorganisms could potentially survive conditions comparable to those in near-Earth space, bridging the fields of aerobiology and astrobiology. As space exploration advanced, interest in microbial behavior under microgravity increased. Microgravity alters fundamental biological processes, including gene expression, metabolism, and virulence in microorganisms. Studies involving Escherichia coli and Salmonella enterica have revealed that reduced gravity conditions can enhance growth rates and virulence factor expression, posing new challenges for astronaut health (Nickerson et al., 2004; Baker et al., 2004). Similarly, Abshire et al. (2016) reported transcriptomic changes in Mycobacterium marinum exposed to simulated microgravity, resulting in enhanced resistance to stress. These findings underscore how microgravity acts as an environmental signal that reprograms microbial physiology. Ground-based experiments using rotating-wall vessels and clinostats have been instrumental in modeling these effects (Hammond & Hammond, 2001; Dedolph et al., 1967). Microbial studies conducted aboard the International Space Station (ISS) have deepened understanding of microbial diversity and resilience in closed environments. The ISS acts as a controlled ecosystem where microorganisms are continuously introduced through cargo, crew, and supplies. Over time, the ISS has become a “microbial observatory,” revealing how microorganisms adapt, colonize, and interact within spacecraft habitats (Checinska Sielaff et al., 2019). These microorganisms include both benign and potentially pathogenic species, such as Staphylococcus aureus and Enterococcus faecalis, which have been found to form biofilms on various surfaces (Bryan et al., 2021). Such biofilms can lead to material degradation, clog filtration systems, and pose health risks to astronauts (Pierson et al., 2013; Khodadad et al., 2021).
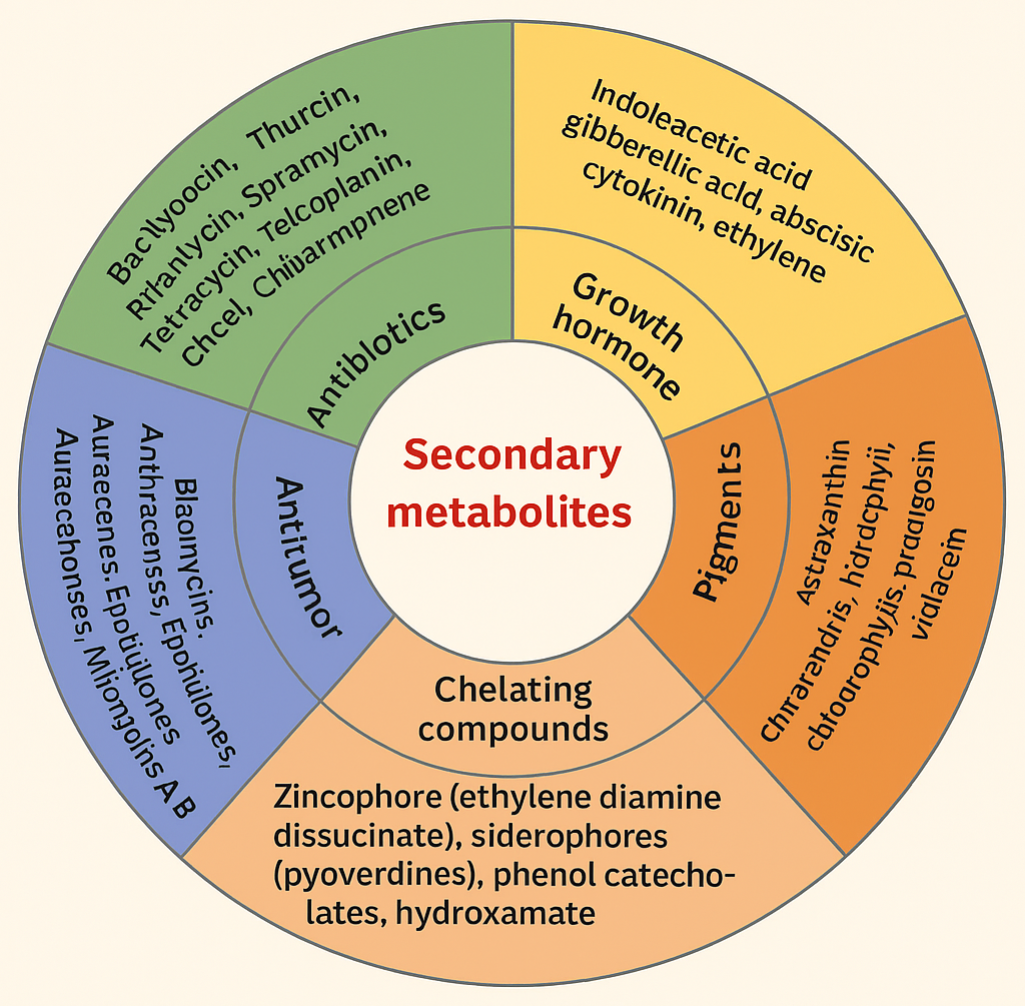
Research has also demonstrated that some microbes not only endure spaceflight but also maintain or increase their metabolic activity. For example, certain bacteria and fungi can produce secondary metabolites, such as antibiotics, in altered quantities under spaceflight conditions (Gao et al., 2011). Furthermore, extremophiles like Deinococcus radiodurans and Bacillus subtilis have exhibited extraordinary survival rates under cosmic radiation, lending credence to panspermia theories—the hypothesis that life could spread between planets via microbial spores embedded in meteoroids or spacecraft (Fajardo-Cavazos et al., 2005; Kawaguchi et al., 2016). The Tanpopo Mission on the ISS further validated these hypotheses by exposing microbial aggregates to space for extended periods and demonstrating their potential to withstand UV and cosmic radiation (Kawaguchi et al., 2013; Kawaguchi et al., 2016). Such studies are essential not only for astrobiology but also for planetary protection, as recommended by the National Research Council (2006), which emphasizes preventing forward and backward contamination between Earth and other celestial bodies. In addition to the risks microbes pose in space, they also hold promise as allies in sustaining long-term human missions. Microorganisms are being investigated for their applications in biomining, waste recycling, and microbial fuel cells, which can generate energy from organic waste (Cockell et al., 2020; De Vet & Rutgers, 2007). The European Space Agency’s MELiSSA project, for instance, explores the integration of microbial consortia into closed-loop life support systems that recycle waste into oxygen, water, and nutrients, supporting sustainable space habitation (Cockell & Horneck, 2004). This study therefore aims to explore how microorganisms adapt and survive in space environments, focusing on their implications for astronaut health, spacecraft contamination, and mission success. The objectives are to examine microbial responses to space-related stressors such as cosmic radiation, microgravity, and confined habitats; to investigate the potential for biofilm formation and material degradation in spacecraft systems; and to evaluate risks posed by increased virulence or resistance. Ultimately, understanding these adaptive mechanisms will aid in developing effective countermeasures—such as antimicrobial coatings, sterilization techniques, and biofilm-resistant materials—to ensure safe, sustainable, and contamination-free human space exploration.