1. Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disorder that affects approximately 2–3% of the global population, making it one of the most common autoimmune diseases worldwide (Di Meglio, Villanova, & Nestle, 2014). Characterized by erythematous, scaly plaques primarily affecting the scalp, elbows, and knees, psoriasis is not only a dermatological concern but also a systemic inflammatory condition with profound psychological and physiological impacts. Traditional perspectives have long focused on the skin itself; however, recent research indicates that the roots of psoriasis may extend deeper—into the gut (Lowes, Bowcock, & Krueger, 2007).
The human gut is home to trillions of microorganisms collectively known as the gut microbiota. These microbes play a critical role in maintaining immune balance, nutrient absorption, and metabolic homeostasis (Belkaid & Hand, 2014). Disruption of this delicate ecosystem, known as dysbiosis, has been implicated in a wide range of autoimmune and inflammatory conditions such as inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis (Figure 1) (Furusawa et al., 2013; Zhang et al., 2015). The discovery that psoriasis shares similar immunological pathways with these diseases has prompted increasing attention toward the gut–skin connection as a crucial factor in its pathogenesis (Schade, Gonzales, & Laughlin, 2016).
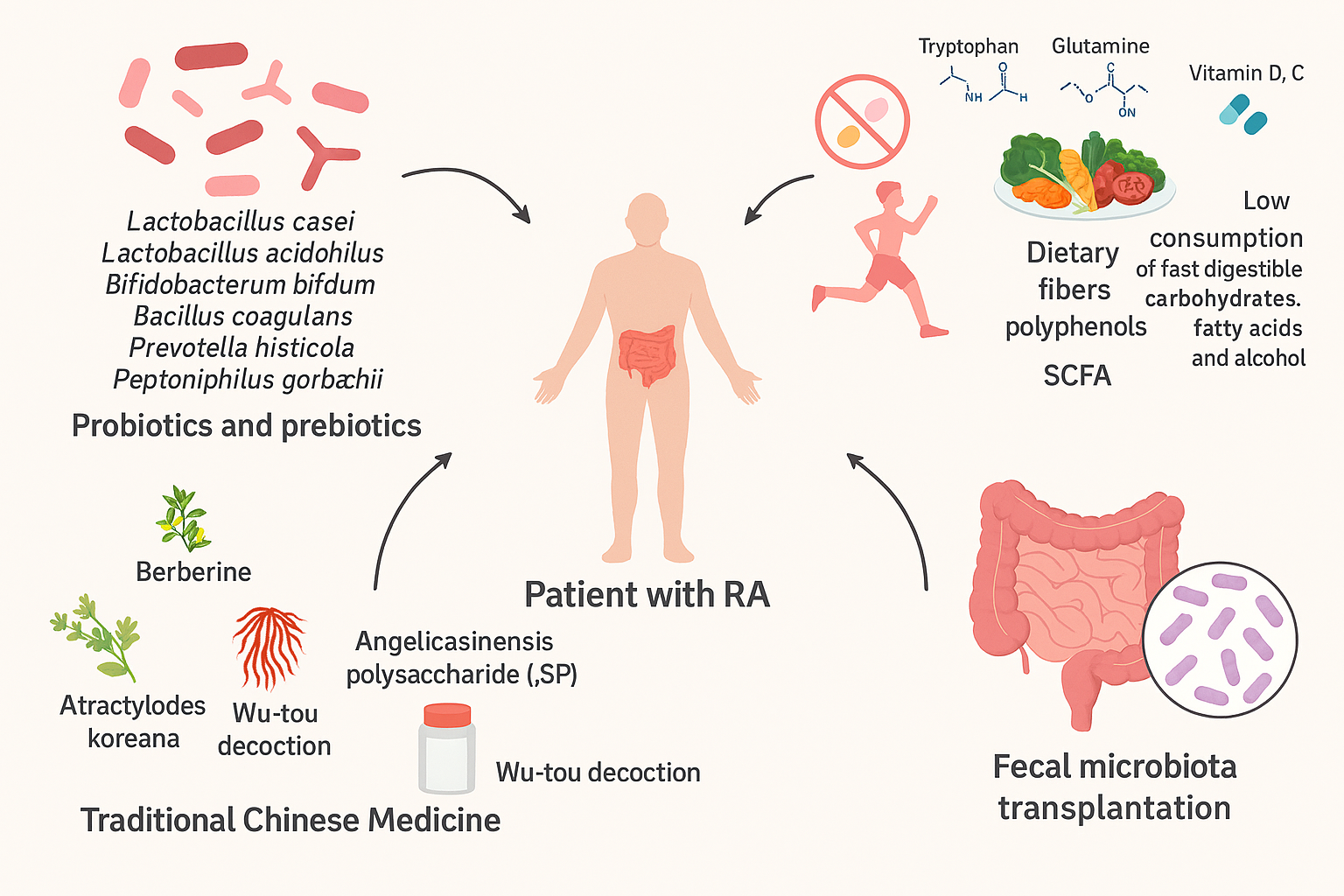
Figure 1: A Novel Strategy for Rheumatoid Arthritis Therapy. Gut Microbiota Interventions for Rheumatoid Arthritis (RA) to modulate gut microbiota in patients with RA. Key approaches include: Probiotics and Prebiotics: Specific strains such as Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium bifidum, Bacillus coagulans, Prevotella histicola, and Peptoniphilus gorbachii. Diet and Lifestyle: Incorporating dietary fibers, polyphenols, short-chain fatty acids (SCFA), essential amino acids (tryptophan, glutamine), vitamins D and C, and reducing fast-digestible carbohydrates, fatty acids, and alcohol. Traditional Chinese Medicine: Use of berberine, Atractylodes koreana, Angelica sinensis polysaccharide (ASP), and Wu-tou decoction. Fecal Microbiota Transplantation: Restoring gut microbial balance to improve systemic health. (courtesy of image from Zhang et al., (2015)
Studies show that individuals with psoriasis often exhibit significant alterations in gut microbial diversity and composition. Beneficial bacteria such as Lactobacillus and Bifidobacterium, known for maintaining intestinal barrier integrity, are often reduced in psoriatic patients (Codoñer et al., 2016; Hidalgo-Cantabrana et al., 2017). Conversely, potentially pathogenic bacteria, including Escherichia coli, tend to increase, promoting systemic inflammation and immune dysregulation (Schade et al., 2016). This imbalance compromises the intestinal barrier—a phenomenon commonly referred to as “leaky gut”—allowing microbial products like lipopolysaccharides to enter the bloodstream, triggering an immune response that can exacerbate psoriatic inflammation (Fasano, 2012).
The gut–skin axis is a bidirectional communication system that links intestinal microbiota and skin health through immune, endocrine, and metabolic pathways (Grice & Segre, 2011). It provides a compelling explanation for how intestinal dysbiosis can manifest as cutaneous inflammation. For instance, Toll-like receptors (TLRs), essential for microbial recognition, play a significant role in modulating immune responses; overactivation of these receptors has been linked to chronic inflammatory diseases, including psoriasis (O’Neill, Golenbock, & Bowie, 2013). Similarly, metabolites produced by commensal bacteria—such as short-chain fatty acids—support the differentiation of regulatory T cells, which are key in maintaining immune tolerance (Arpaia et al., 2013). When this microbial signaling network is disrupted, systemic inflammation becomes amplified, contributing to the persistence and severity of psoriatic lesions (Eppinga et al., 2016).
Beyond microbiota composition, lifestyle and dietary factors further influence the relationship between gut health and psoriasis. Adherence to anti-inflammatory diets, such as the Mediterranean diet, has been associated with milder psoriasis severity due to its positive impact on gut microbial balance and reduction in oxidative stress (Barrea et al., 2015). Conversely, Western dietary patterns rich in processed foods and saturated fats can promote gut dysbiosis, impair immune regulation, and intensify skin inflammation (Briganti & Picardo, 2003). This evidence underscores the integrative nature of psoriasis management—where nutrition, gut microbiota, and immune health interact in a dynamic, interdependent system.
Given the growing recognition of the gut’s role in systemic immunity, probiotics—live microorganisms that confer health benefits when consumed in adequate amounts—have emerged as a promising therapeutic option. Probiotics have demonstrated the ability to modulate immune responses, reinforce intestinal barrier function, and suppress inflammatory cytokines that drive psoriatic pathology (Borody & Khoruts, 2012). In particular, probiotic strains such as Lactobacillus and Bifidobacterium may restore microbial diversity and enhance immune tolerance, offering potential relief for psoriatic patients resistant to conventional therapies (Ghoreschi, Balato, Enerbäck, & Sabat, 2017).
Recent advances in immunology and microbiome science support a paradigm shift from surface-level symptom management toward systemic restoration of health. Rather than viewing psoriasis merely as a skin disease, current evidence positions it as a complex systemic disorder influenced by microbial and immune interactions (Furusawa et al., 2013). Restoring gut microbial balance through probiotic supplementation, dietary interventions, and lifestyle modifications could therefore represent a more holistic and sustainable strategy for managing psoriasis.
This systematic review aims to synthesize the emerging evidence connecting gut dysbiosis and psoriasis while examining the therapeutic potential of probiotics in restoring immune balance. By integrating findings from dermatology, microbiology, and immunology, the review highlights how the gut–skin axis could serve as a therapeutic target for long-term psoriasis management. Understanding this connection not only redefines how psoriasis is treated but also transforms the broader perspective of health—from isolating symptoms to nurturing systemic harmony (Figure 2).
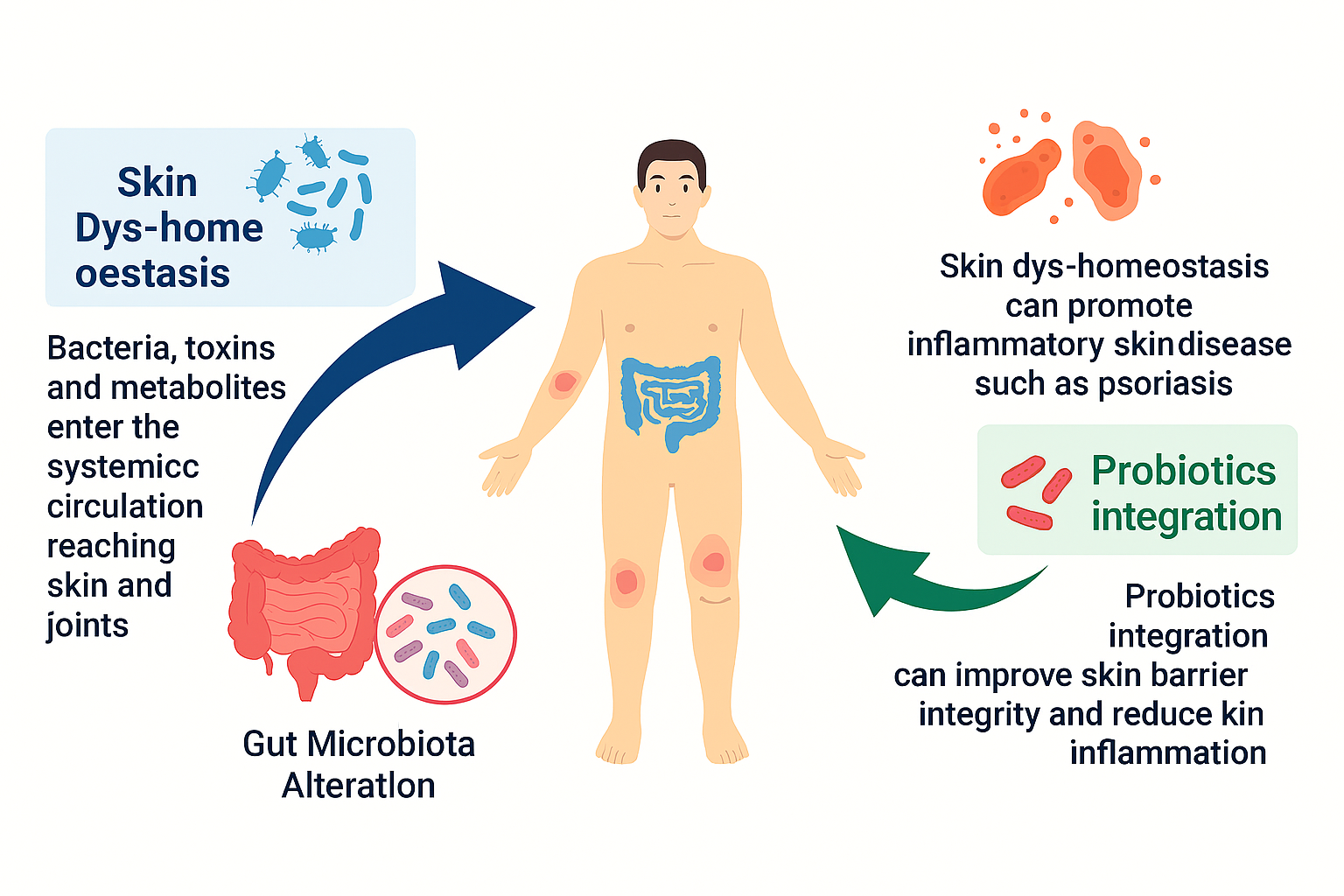
Figure 2. The Skin Microbiome and its Role in Psoriasis. Gut-Skin Axis and Inflammation to demonstrates the relationship between gut microbiota and skin health. Gut Microbiota Alteration: Disruption in gut microbial composition allows bacteria, toxins, and metabolites to enter systemic circulation, affecting skin and joints. Skin Dys-homeostasis: Imbalance in skin microbiota can promote inflammatory skin diseases such as psoriasis. Probiotics Integration: Introducing beneficial microbes can enhance skin barrier integrity and reduce skin inflammation. (Courtesy of image from Grice, E. A., & Segre, J. A. (2011)
