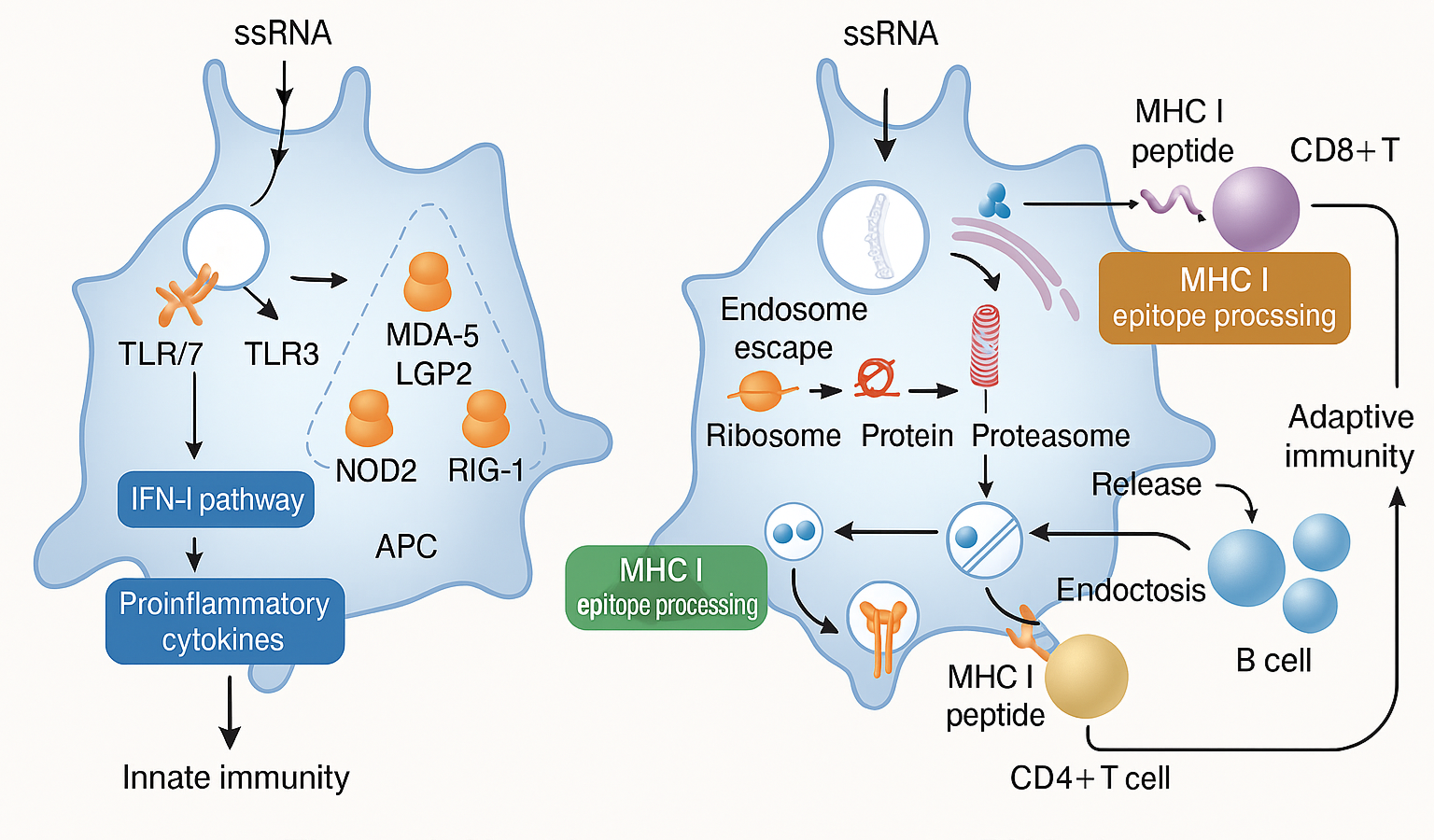
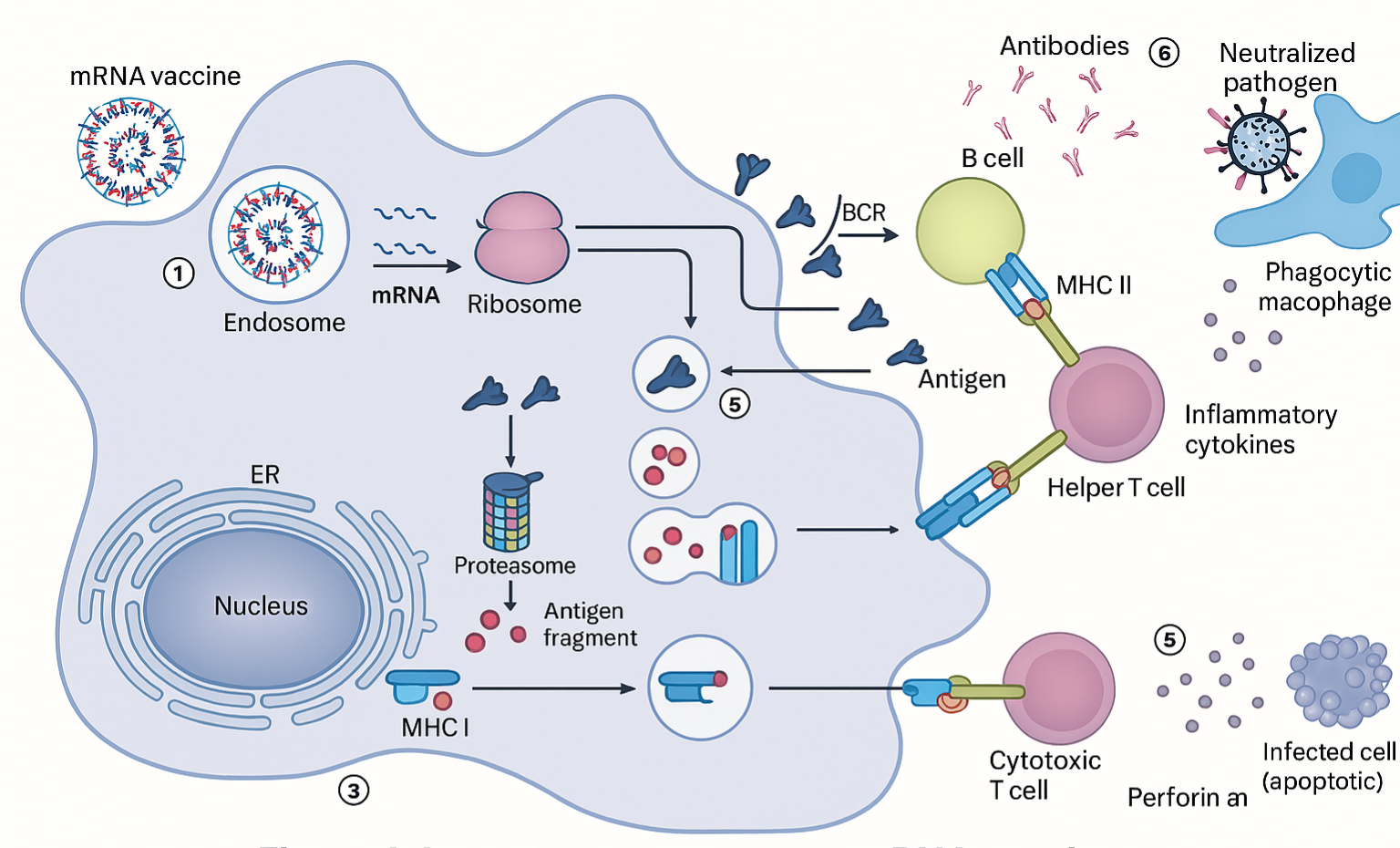
The composition of mRNA vaccines plays a crucial role in determining their immunogenicity and potential interactions with the gut microbiota. Unlike traditional vaccines that use live or inactivated pathogens, mRNA vaccines rely on synthetic lipid nanoparticles (LNPs) to deliver genetic instructions to host cells. While these components are designed to be safe and effective, they may have unintended effects on gut microbial populations through immune modulation, lipid metabolism alterations, and direct microbial interactions. This section explores how mRNA vaccine components, including lipid nanoparticles, polyethylene glycol (PEG), and stabilizing agents, influence gut microbiota balance and function (Table 3).
Table 3. Summary of Gut Microbiota’s Role in Vaccine Efficacy and Immune Regulation
|
Microbial Factor
|
Associated Immune Outcome
|
Supporting Evidence (Pre-2018)
|
Implications for mRNA Vaccine Efficacy
|
|
High Microbial Diversity
|
Stronger antibody and T-cell responses
|
Harris et al. (2017); Steed et al. (2017)
|
Diverse microbiota enhances adaptive immunity post-mRNA vaccination.
|
|
Dominance of SCFA-Producing Bacteria (Bifidobacterium, Lactobacillus)
|
Enhanced mucosal immunity, reduced inflammation
|
Honda & Littman (2016); de Groot & van den Broek (2017)
|
Promotes optimal immune memory and tolerance to vaccination.
|
|
Dysbiosis or Reduced Diversity
|
Weakened vaccine response, impaired antibody production
|
Valdez et al. (2014); Belkaid & Hand (2014)
|
Gut imbalance may reduce mRNA vaccine immunogenicity in vulnerable groups.
|
|
Antibiotic-Induced Microbiome Disruption
|
Lower antibody titers post-vaccination
|
Valdez et al. (2014)
|
Avoiding antibiotics before vaccination may improve immune response.
|
|
Probiotic and Prebiotic Interventions
|
Improved antibody levels and reduced side effects
|
de Groot & van den Broek (2017); Steed et al. (2017)
|
Probiotic support may enhance mRNA vaccine tolerance and immune strength.
|
Lipid Nanoparticles and Their Effects on Gut MicrobiotaLipid nanoparticles (LNPs) are essential for the delivery of mRNA into human cells. They protect the fragile mRNA molecules from degradation while facilitating efficient uptake by immune cells (Pardi et al., 2018). However, LNPs consist of synthetic lipids that may interact with biological membranes, immune receptors, and microbial populations in the gut.
research demonstrated that dietary and synthetic lipids could alter gut microbiota composition by selectively promoting or inhibiting bacterial growth (Devkota et al., 2012). Studies found that certain emulsifiers and lipid-based compounds could disrupt the mucus layer of the gut, leading to microbiome imbalances and increased intestinal permeability (Chassaing et al., 2015). While LNPs used in mRNA vaccines differ from dietary lipids, they share structural similarities that might influence gut microbial ecology.One potential concern is the effect of LNPs on gut-resident immune cells. Pre-2018 studies on immune system–microbiome interactions showed that lipids could activate dendritic cells and macrophages, leading to shifts in microbial composition (Ghosh et al., 2013). If LNPs trigger localized immune responses in the gut, they may create an environment that favors pro-inflammatory bacterial species such as Enterobacteriaceae while reducing beneficial microbes like Bifidobacterium and Lactobacillus.Additionally, lipid metabolism plays a crucial role in microbial diversity. Research prior to 2018 highlighted the connection between lipid-rich environments and the expansion of specific bacterial species linked to metabolic disorders (Turnbaugh et al., 2009). Since LNPs introduce novel lipid structures into the body, their influence on gut microbiota composition warrants further investigation.
7.1 Polyethylene Glycol (PEG) and Microbial Dysbiosis
Polyethylene glycol (PEG) is commonly used as a stabilizing agent in mRNA vaccines to enhance nanoparticle stability and prolong shelf life. While PEG is generally regarded as safe for human consumption and medical use, pre-2018 studies raised concerns about its potential impact on gut microbiota, particularly in individuals with sensitivities or compromised gut health.PEG is widely used in laxatives and pharmaceutical formulations, where it influences water retention and gut motility (Huttenhower et al., 2012). However, chronic exposure to PEG-containing substances has been associated with microbiome disruptions. A study on PEG-based laxatives found that prolonged use led to decreased microbial diversity and selective depletion of beneficial bacteria such as Faecalibacterium prausnitzii (Mancabelli et al., 2017).
Furthermore, PEG can modulate gut permeability, affecting the tight junctions that maintain intestinal barrier integrity. Pre-2018 research suggested that disruptions in gut permeability could lead to increased translocation of bacterial metabolites, potentially triggering low-grade systemic inflammation (Bischoff et al., 2014). Since mRNA vaccines contain PEG as part of their nanoparticle formulation, further studies are needed to determine whether repeated exposure contributes to microbiome shifts, particularly in individuals with pre-existing gut conditions.
7.2 Stabilizing Agents and Their Influence on Gut Microbial Composition
In addition to lipids and PEG, mRNA vaccines contain stabilizing agents, including salts, buffers, and cryoprotectants, to preserve vaccine integrity. While these components are typically used in small amounts, their effects on gut microbiota should not be overlooked.
Studies on pharmaceutical excipients found that certain stabilizing agents, such as polysorbates and sugar-based cryoprotectants, could influence bacterial adhesion and biofilm formation (Ruas-Madiedo et al., 2011). Polysorbates, for example, were shown to alter microbial growth patterns by disrupting bacterial membranes, potentially leading to shifts in gut microbial balance (Chassaing et al., 2015).Furthermore, buffers used in vaccine formulations, such as phosphate-based compounds, may influence microbial metabolism. Research suggested that phosphate availability could regulate microbial diversity by influencing the growth of specific bacterial groups (White & Hager, 2013). While the concentrations used in vaccines are relatively low, repeated exposure may have cumulative effects on gut microbiota, particularly in individuals receiving multiple booster doses.
7.3 Potential Microbial Adaptations to Vaccine Components
Microbial communities are highly adaptable and can develop mechanisms to metabolize foreign compounds, including vaccine components. Studies on gut microbiota adaptability demonstrated that bacteria could acquire metabolic pathways to process novel dietary and pharmaceutical compounds, leading to shifts in microbial function (Ley et al., 2006).For instance, research on antibiotic exposure revealed that certain gut bacteria developed resistance mechanisms, altering their metabolic activity in response to repeated exposure (Dethlefsen et al., 2008). Similarly, gut microbes may adapt to recurring exposure to vaccine-related compounds, potentially leading to long-term changes in microbial ecology.Additionally, vaccine-induced immune activation can indirectly shape microbial communities by altering host metabolic processes. Studies on immune-mediated microbiome interactions found that systemic inflammation influenced gut microbial composition by modifying bile acid metabolism and nutrient availability (Tremaroli & Bäckhed, 2012). Since mRNA vaccines elicit strong immune responses, they may contribute to subtle but lasting shifts in microbial populations over time
7.4 Implications for Long-Term Gut Health and Microbiome Resilience
Understanding the impact of mRNA vaccine components on gut microbiota is crucial for optimizing vaccine safety and minimizing potential side effects. While most individuals experience no significant disruptions in gut health following vaccination, those with pre-existing microbiome imbalances may be more susceptible to vaccine-induced microbial shifts.To promote gut resilience and mitigate potential microbiome disruptions, several strategies can be considered:
Incorporating Gut-Protective Nutrients:
Consuming foods rich in prebiotics and polyphenols can support microbial diversity and enhance gut barrier function. Pre-2018 studies highlighted the benefits of polyphenols from green tea, berries, and dark chocolate in modulating gut microbiota (Etxeberria et al., 2015).
Monitoring Lipid Intake and Metabolism:
Since LNPs introduce synthetic lipids into the body, maintaining a balanced dietary fat intake may help regulate lipid metabolism and prevent microbiome imbalances. Omega-3 fatty acids, in particular, have been shown to counteract inflammation-induced microbiota shifts (Costantini et al., 2017).
Supporting Detoxification Pathways:
Enhancing liver and gut detoxification through adequate hydration, fiber intake, and herbal supplements such as milk thistle and dandelion root may help the body process and eliminate vaccine components efficiently (de Groot et al., 2017).
Personalized Microbiome Monitoring:
Individuals with gut-related concerns may benefit from microbiome testing before and after vaccination to assess potential shifts in microbial composition. Advances in gut microbiome sequencing have enabled personalized dietary and probiotic interventions based on individual microbial profiles (Huttenhower et al., 2012).