1. Introduction
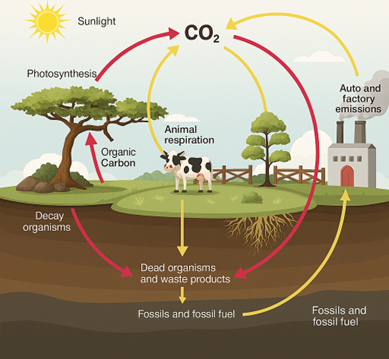
Nature possesses an extraordinary ability to decompose organic matter through biological processes, ensuring the continuous cycling of nutrients within ecosystems. This natural recycling mechanism, known as biodegradation, involves the breakdown of organic materials by microorganisms such as bacteria, fungi, and archaea (Ågren & Bossatta, 1996; Chapman & Gray, 1981). It is a fundamental ecological process that maintains balance by preventing the excessive accumulation of organic waste. Without biodegradation, ecosystems would be overwhelmed by layers of decaying material, rendering life unsustainable.
Biodegradation is primarily driven by microbial metabolism, where microorganisms utilize complex organic compounds as energy and carbon sources, converting them into simpler molecules. The process occurs in two major forms: aerobic and anaerobic biodegradation. Aerobic degradation requires oxygen and typically results in the formation of carbon dioxide, water, and biomass, whereas anaerobic degradation occurs in oxygen-deprived environments and produces methane, carbon dioxide, and organic acids (Firestone & Davidson, 1989; Cooper & Smith, 1963). Both pathways are essential in maintaining the natural balance of carbon and nutrient cycles in soil, aquatic systems, and waste management environments (Carter, 2001; Bellamy et al., 2005).
A central function of biodegradation lies in nutrient cycling, which transforms organic matter into elemental forms vital for biological productivity. The decomposition process releases essential nutrients such as carbon, nitrogen, phosphorus, and sulfur, sustaining plant growth and microbial activity (Barraclough, 1997; Cordell et al., 2009). These nutrient transformations are integral to ecosystem sustainability, influencing soil structure, fertility, and agricultural yield (Clement & Williams, 1962; Campbell et al., 1967). Organic matter degradation by soil microorganisms, including bacteria, protozoa, and nematodes, supports the dynamic equilibrium of terrestrial ecosystems (Alphei et al., 1996; Coûteaux et al., 1995).
However, the natural efficiency of biodegradation is increasingly challenged by anthropogenic activities. The introduction of synthetic materials such as plastics, petroleum-based polymers, and industrial chemicals has significantly altered the balance of natural decomposition processes (De Wilde & De Baere, 1998; Avella et al., 2000). Unlike natural organic matter, which typically decomposes within weeks or months, synthetic pollutants persist for decades or even centuries, leading to environmental accumulation and ecological disruption (Witt et al., 2001; Pagga et al., 1995). Persistent plastics, for instance, contribute to the formation of microplastics that infiltrate aquatic systems, affecting marine organisms and entering the food web (Iovino et al., 2008; Kijchavengkul et al., 2008).
In addition to plastics, waste management practices such as composting and landfill operations have been identified as significant contributors to greenhouse gas emissions, particularly methane and nitrous oxide, when biodegradation occurs under uncontrolled conditions (Amlinger et al., 2002; Andersen et al., 2010). Studies indicate that microbial degradation in composting systems not only contributes to organic waste stabilization but also plays a role in climate-relevant gas emissions (Amlinger et al., 2008; Boldrin et al., 2009). The efficiency of biodegradation in these systems depends heavily on factors such as temperature, oxygen availability, moisture, and the composition of microbial communities (Dickinson et al., 1981; Dungait et al., 2010).
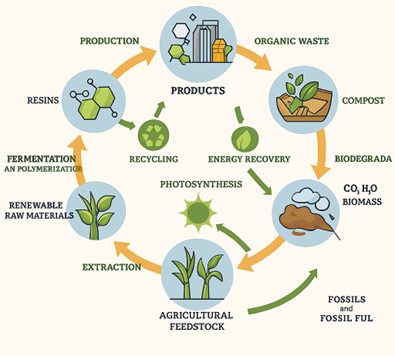
Given these challenges, researchers are increasingly focusing on enhancing biodegradation processes to promote sustainable waste management and environmental conservation. Bioremediation—the use of living microorganisms to degrade or detoxify environmental contaminants—has emerged as an effective strategy for mitigating pollution (Gregorich et al., 2003; Ramirez et al., 2006). Through optimized environmental conditions and microbial consortia, bioremediation accelerates the breakdown of organic and synthetic compounds, reducing their ecological footprint (Brinkmann et al., 2000; Dungait et al., 2009). Advances in molecular biology and microbial genetics further enable scientists to engineer microorganisms with enhanced enzymatic capabilities for degrading persistent materials such as polyesters and polyhydroxyalkanoates (Avella et al., 2000; Witt et al., 2001).
Furthermore, the development of biodegradable polymers compatible with composting systems offers a promising direction for sustainable material design. Research on aliphatic–aromatic copolyesters, poly(lactic acid) composites, and starch-based blends has demonstrated their potential for accelerated biodegradation under controlled environmental conditions (Iovino et al., 2008; De Wilde & De Baere, 1998). These materials not only reduce the persistence of plastic waste but also integrate more effectively into natural decomposition pathways.
Understanding the mechanisms, environmental variables, and limitations of biodegradation is essential for designing sustainable ecosystems that minimize waste and pollution. Through the synergistic integration of natural microbial processes, engineered solutions, and eco-friendly materials, biodegradation can serve as a cornerstone for addressing global waste management challenges. Continued research into microbial ecology, stable isotope tracing, and carbon cycling dynamics provides valuable insights into optimizing biodegradation efficiency in both natural and engineered systems (Dungait & Bol, 2005; Dungait et al., 2008). By deepening our understanding of these complex biological processes, scientists and policymakers can develop innovative strategies to restore ecological balance, enhance soil fertility, and promote environmental sustainability worldwide.
Nature possesses an extraordinary ability to decompose organic matter through biological processes, ensuring the continuous cycling of nutrients within ecosystems. This natural recycling mechanism, known as biodegradation, involves the breakdown of organic materials by microorganisms such as bacteria, fungi, and archaea (Ågren & Bossatta, 1996; Chapman & Gray, 1981). It is a fundamental ecological process that maintains balance by preventing the excessive accumulation of organic waste. Without biodegradation, ecosystems would be overwhelmed by layers of decaying material, rendering life unsustainable.
Biodegradation is primarily driven by microbial metabolism, where microorganisms utilize complex organic compounds as energy and carbon sources, converting them into simpler molecules. The process occurs in two major forms: aerobic and anaerobic biodegradation. Aerobic degradation requires oxygen and typically results in the formation of carbon dioxide, water, and biomass, whereas anaerobic degradation occurs in oxygen-deprived environments and produces methane, carbon dioxide, and organic acids (Firestone & Davidson, 1989; Cooper & Smith, 1963). Both pathways are essential in maintaining the natural balance of carbon and nutrient cycles in soil, aquatic systems, and waste management environments (Carter, 2001; Bellamy et al., 2005).
A central function of biodegradation lies in nutrient cycling, which transforms organic matter into elemental forms vital for biological productivity. The decomposition process releases essential nutrients such as carbon, nitrogen, phosphorus, and sulfur, sustaining plant growth and microbial activity (Barraclough, 1997; Cordell et al., 2009). These nutrient transformations are integral to ecosystem sustainability, influencing soil structure, fertility, and agricultural yield (Clement & Williams, 1962; Campbell et al., 1967). Organic matter degradation by soil microorganisms, including bacteria, protozoa, and nematodes, supports the dynamic equilibrium of terrestrial ecosystems (Alphei et al., 1996; Coûteaux et al., 1995).
However, the natural efficiency of biodegradation is increasingly challenged by anthropogenic activities. The introduction of synthetic materials such as plastics, petroleum-based polymers, and industrial chemicals has significantly altered the balance of natural decomposition processes (De Wilde & De Baere, 1998; Avella et al., 2000). Unlike natural organic matter, which typically decomposes within weeks or months, synthetic pollutants persist for decades or even centuries, leading to environmental accumulation and ecological disruption (Witt et al., 2001; Pagga et al., 1995). Persistent plastics, for instance, contribute to the formation of microplastics that infiltrate aquatic systems, affecting marine organisms and entering the food web (Iovino et al., 2008; Kijchavengkul et al., 2008).
In addition to plastics, waste management practices such as composting and landfill operations have been identified as significant contributors to greenhouse gas emissions, particularly methane and nitrous oxide, when biodegradation occurs under uncontrolled conditions (Amlinger et al., 2002; Andersen et al., 2010). Studies indicate that microbial degradation in composting systems not only contributes to organic waste stabilization but also plays a role in climate-relevant gas emissions (Amlinger et al., 2008; Boldrin et al., 2009). The efficiency of biodegradation in these systems depends heavily on factors such as temperature, oxygen availability, moisture, and the composition of microbial communities (Dickinson et al., 1981; Dungait et al., 2010).
Given these challenges, researchers are increasingly focusing on enhancing biodegradation processes to promote sustainable waste management and environmental conservation. Bioremediation—the use of living microorganisms to degrade or detoxify environmental contaminants—has emerged as an effective strategy for mitigating pollution (Gregorich et al., 2003; Ramirez et al., 2006). Through optimized environmental conditions and microbial consortia, bioremediation accelerates the breakdown of organic and synthetic compounds, reducing their ecological footprint (Brinkmann et al., 2000; Dungait et al., 2009). Advances in molecular biology and microbial genetics further enable scientists to engineer microorganisms with enhanced enzymatic capabilities for degrading persistent materials such as polyesters and polyhydroxyalkanoates (Avella et al., 2000; Witt et al., 2001).
Furthermore, the development of biodegradable polymers compatible with composting systems offers a promising direction for sustainable material design. Research on aliphatic–aromatic copolyesters, poly(lactic acid) composites, and starch-based blends has demonstrated their potential for accelerated biodegradation under controlled environmental conditions (Iovino et al., 2008; De Wilde & De Baere, 1998). These materials not only reduce the persistence of plastic waste but also integrate more effectively into natural decomposition pathways.
Understanding the mechanisms, environmental variables, and limitations of biodegradation is essential for designing sustainable ecosystems that minimize waste and pollution. Through the synergistic integration of natural microbial processes, engineered solutions, and eco-friendly materials, biodegradation can serve as a cornerstone for addressing global waste management challenges. Continued research into microbial ecology, stable isotope tracing, and carbon cycling dynamics provides valuable insights into optimizing biodegradation efficiency in both natural and engineered systems (Dungait & Bol, 2005; Dungait et al., 2008). By deepening our understanding of these complex biological processes, scientists and policymakers can develop innovative strategies to restore ecological balance, enhance soil fertility, and promote environmental sustainability worldwide.